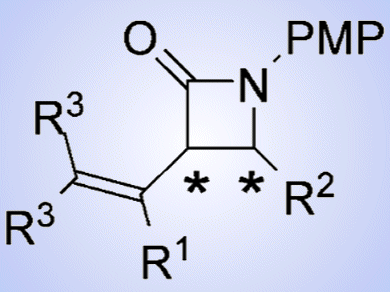
β-Lactams are one of the basic skeletons of antibiotics and have been clinically used since the 1940s. Penicillin, cephalosporin, thienamycin, and 1β-methyl carbapenem are the representative examples of β-lactam antibiotics. As the stereochemistry of the substituents at the 3 and 4 positions of the β-lactam ring affects the bioactivity, numerous methods for the stereocontrolled synthesis of 3,4-cis– and trans-substituted β-lactams have been attempted. Among them, a stereoselective synthesis of these isomers from a single precursor is highly desired.
Makoto Shimizu and co-workers, Mie University, Japan, developed a highly enantioselective synthesis of both 3,4-cis– and trans-β-lactams by the reduction of iminocyclobutenones catalyzed by a chiral phosphoric acid followed by thermal rearrangement of the resulting aminocyclobutenones. This chiral β-lactam synthesis is particularly attractive because four possible enantiomers of 3,4-disubstituted β-lactams can be synthesized from a single iminocyclobutenone by using both enantiomers of the chiral phosphoric acid catalyst in the enantioselective reduction and appropriate amines in the subsequent thermal rearrangement reaction.
- Chiral β-Lactam Synthesis through the Enantioselective Reduction of Iminocyclobutenones and the Thermal Rearrangement of Aminocyclobutenones,
Iwao Hachiya, Akinori Ito, Makoto Shimizu,
Asian J. Org. Chem. 2014, 3, 614–618.
DOI: 10.1002/ajoc.201402005