Enzymatically Active Pepsin Membranes
Novel enzymatically active membranes can complete two steps in one: they can enzymatically split proteins and simultaneously separate the products. The membranes are produced by cross-linking pepsin on a porous support, a simple process that is also applicable to other enzymes and on an industrial scale. A team of Dutch and German scientists report their findings in the journal Angewandte Chemie.
Pepsin is an important digestive enzyme. It is a protease, an enzyme that breaks apart peptide bonds, chopping protein molecules in our intestines into smaller peptide fragments. Proteases are also used in the food industry, for the production of cheese or the removal of cloudiness from beverages like wine, beer, and fruit juice. Special proteases are also used in the production hypoallergenic food: They break down target allergenic proteins.
Reusable Cross-Linked Enzymes on a Polymer Substrate
When they are used in their dissolved form, proteases have a number of disadvantages: the enzymes are not stable because they can break each other down. They cannot be reused though, which drives up the cost. Soluble enzymes must also not remain in the treated beverages; indeed, the German beer purity law forbids this. When the enzymes are immobilized, on the other hand, they can be recovered and reused multiple times.
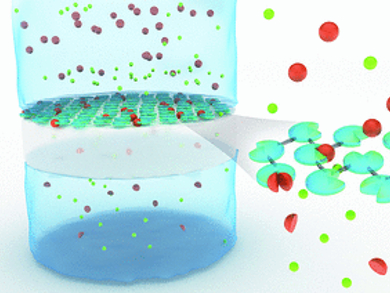
Immobilization can be achieved by fixing the enzymes on a substrate or by cross-linking them. A team headed by Nieck E. Benes, University of Twente, Enschede, Netherlands, and Matthias Wessling, RWTH Aachen and DWI – Leibniz Institute for Interactive Materials, Aachen, Gemany, has now developed a simple method that unifies these two principles: the researchers coat an ultrathin, porous polymer membrane with protease and then use the reagent trimesoyl chloride to initiate cross-linking of the molecules. This causes the pepsin molecules to be so tightly linked that they form a homogenous film. The pepsin molecules maintain their ability to break down substrates under acidic conditions, and their activity continues for a long time because they cannot digest each other.
Large molecules are held back by the 50 to 150 nm thick pepsin membranes, while smaller degradation products quickly pass through. This makes the products easy to separate and a continuous process can be established.
- Enzymatically Active Ultrathin Pepsin Membranes,
Michiel J. T. Raaijmakers, Thomas Schmidt, Monika Barth, Murat Tutus, Nieck E. Benes, Matthias Wessling,
Angew. Chem. Int. Ed. 2015.
DOI: 10.1002/anie.201411263