Photochemical CO2 reduction could lead to solutions for current energy and environmental problems. Various types of metal complexes have been studied as catalysts. Ruthenium mono- and bis(bipyridyl)dicarbonyl complexes have recently received renewed interest as reduction terminal catalysts of artificial photosynthetic systems containing a semiconductor.
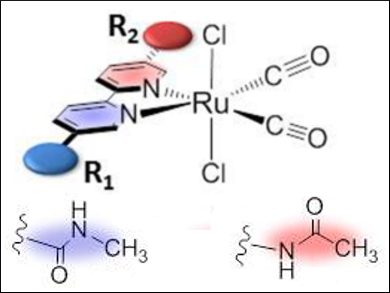
Hitoshi Ishida and co-workers, Kitasato University, Japan, have synthesized a series of trans-(Cl)-[Ru(L)(CO)2Cl2]-type complexes, in which the ligands L are 2,2′-bipyridyl derivatives with amide groups at the 5,5′-positions. The structures of the ruthenium complexes were characterized by NMR spectroscopy, crystallographic analysis, and DFT calculations. The calculations showed that the C-connected amide group (pictured blue) is twisted with respect to the bipyridyl plane, whereas the N-connected amide group (pictured red) is in the plane. This twisted structure raised the energy level of the LUMO (lowest unoccupied molecular orbital) of the complex, resulting in a negative shift of the first reduction potential (Ep) of the ruthenium complex.
The photochemical CO2 reductions catalyzed by the complexes showed that the turnover frequencies (TOFs) were significantly affected by the substituents on the bipyridyl ligand. The TOFs for both CO and formate products increased exponentially as the reduction potential shifted to the negative side down to approximately −1.5 V (vs. Ag/Ag+). Thus, the increase in reaction rates could be systematically explained by the first reduction potential shift, providing a useful method to predict the catalytic activity for CO2 reduction when novel ruthenium catalysts are designed and synthesized.
- trans-(Cl)-[Ru(5,5′-diamide-2,2′-bipyridine)(CO)2Cl2]: Synthesis, Structure and Photocatalytic CO2 Reduction Activity,
Yusuke Kuramochi, Kyohei Fukaya, Makoto Yoshida, Hitoshi Ishida,
Chem. Eur. J. 2015.
DOI: 10.1002/chem.201500782