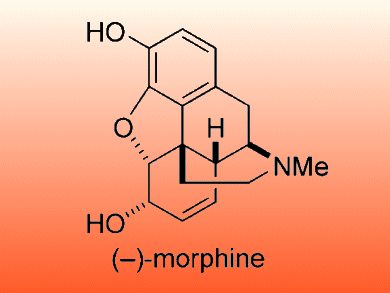
Morphine is an efficient analgesic that is indispensable in cancer pain management; however its addictive nature limits its use. Efforts to make a non-addictive analogue are complicated by the pentacyclic skeleton which has a quaternary carbon.
Tohru Fukuyama and colleagues, University of Tokyo, Japan, report an efficient total synthesis of (–)-morphine. The cyclohexenol unit was prepared by using an enzymatic resolution and a Suzuki–Miyaura coupling. Intramolecular 1,6-addition and aldol reactions were used to form the morphinan core. The formation of the cyclohexanone moiety required ozonolysis followed by an intramolecular aldol condensation as well as an enzymatic resolution.
This approach allowed (–)-morphine to be synthesized in 5 % overall yield with the longest linear sequence consisting of 17 steps.
- Total Synthesis of (–)-Morphine
H. Koizumi, S. Yokoshima, T. Fukuyama,
Chem. Asian J. 2010, 5.
DOI: 10.1002/asia.201000458