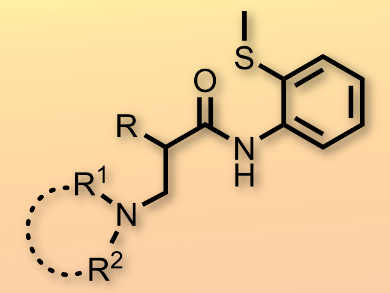
The amination of unactivated C(sp3)–H bonds is challenging and highly desirable, since the resulting products are ubiquitous in pharmaceuticals, agricultural chemicals, and natural products. Jun Qin and colleagues, Yunnan University, China, have accomplished the intermolecular amination of unactivated C(sp3)–H bonds using a PdII catalyst.
The method proceeds with the assistance of a bidentate directing group, 2-aminothioether, and produces a variety of unnatural and functional β2-amino carboxylic acid derivatives in a straightforward manner. The reaction is highly chemoselective and displays a high functional group tolerance, including towards benzyl and silyl ethers. Moreover, heteroaromatics, such as thiophene, which is known to poison catalysts, were compatible.
The utility of the method was demonstrated on a gram-scale towards the synthesis of β2,2-amino carboxylic esters, which are medicinally useful substrates. Kinetic isotope experiments show that the cleavage of the β-C(sp3)–H bond is the rate-limiting step, which implies that the reaction pathway does not involve the α-proton of the substrate. Based on these observations, the researchers propose a PdII/PdIV catalytic cycle.
- PdII-Catalyzed Intermolecular Amination of Unactivated C(sp3)–H Bonds,
Quan Gou, Gang Liu, Zi-Ning Liu, Jun Qin,
Chem Eur. J. 2015.
DOI: 10.1002/chem.201502375