After covering saccharin, the poisonous lead(II) acetate, cyclamate, aspartame, and acesulfame-K, we have a look at a natural low-calorie sweetener: thaumatin.
16. Thaumatin
Thaumatin is a natural sweetener derived from berries of the West African katamfe shrub (Thaumatococcus danielii, of the arrowroot family; see Fig. 22). This plant, native to Ghana, the Ivory Coast, Togo, and Sierra Leone, was certainly known to local inhabitants long before the introduction of cane sugar as a sweetening agent. W. F. Daniell, an English military doctor and amateur botanist, brought the triangular fruit to England in 1839, and despite the long return voyage, it still tasted sweet upon arrival (see Fig. 22) [67].
|
|
|
Figure 22. Fruit from Thaumatococcus daniellii. The West African shrub bears bright red, triangular fruit, containing three black seeds. Only the yellow seed coat (Arillus) incorporates the sweet material. |
The sweet factor in Thaumatococcus danielii turned out to be a mixture of six structurally very similar proteins, designated as thaumatin I, II, III, a, b, and c. Each of the six contains precisely 270 amino acids, and in each, the chain begins and ends with alanine.
Structure
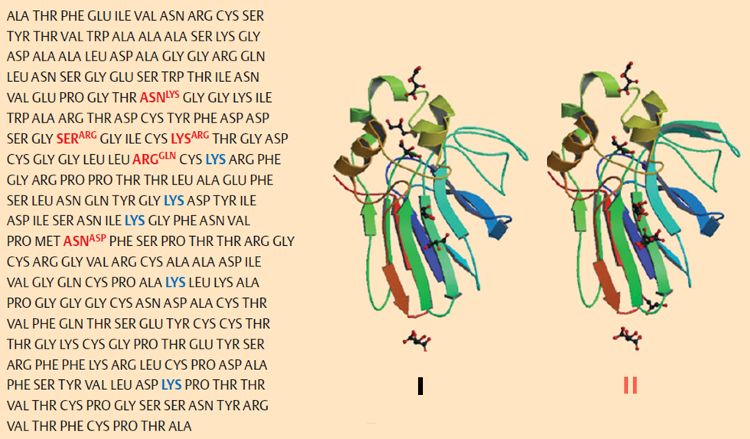
Isolation of the two chief constituents, thaumatin I and thaumatin II, was accomplished in 1972 by chemists at Unilever [68]. The differences between the two amino acid sequences proved to be very slight [69, 70], and X-ray structural analysis revealed their practically identical three-dimensional structures [71] (see Fig. 23). With respect to the thaumatins as sweetening agents, the five lysines 78, 97, 106, 137, and 187 (indicated in blue in Fig. 23) appear to be decisive, since phosphorylation of their side-chain amino groups leads to a diminution in sweetness [72].
|
|
|
Figure 23. Structure of thaumatins I and II [73–75]. Left: The amino acid sequence for thaumatin I, along with the five changes required in this structure for thaumatin II (red). Right: not only are the primary structures for thaumatin I and II very similar, but their three-dimensional structures differ only slightly. |
Sweetening Power
Thaumatin has a sweetening power of 200–3000 relative to saccharose. If one assesses the sweetening power based not on weight, but rather on molecular mass, it turns out that the value is significantly higher: by a factor of 100,000! Interestingly, this sweetener displays a varied taste quality: the sweetness arrives in a delayed fashion, but is then of great endurance and leaves an aftertaste reminiscent of licorice.
Metabolism
Thaumatin is metabolized in the human body just like every other protein nutrient, so that no foreign metabolites can arise. Studies with rats showed that a daily intake of up to 2.8 g/kg body weight caused no adverse effects on the well-being of the animals. With respect to its application as a sweetening agent, the quantities administered were well beyond the powers of imagination, corresponding for an adult to a daily intake of 200 g of thaumatin. From the standpoint of sweetening, this would correspond to 600 kg of saccharose!
No attempt was made to establish an acceptable daily intake (ADI) value for thaumatin, since this would fall outside the limits of any practical application. For pet lovers, it should be pointed out that rats, but also guinea pigs and hamsters, are unable to detect the sweetness of thaumatin, only for this reason are feeding experiments possible at all.
Applications
Thaumatin is marketed under the trade name Talin™, and was approved in Japan as early as 1970, and is used in the EU as food additive E 957. It was granted “generally recognized as safe” (GRAS) status by the US Food and Drug Administration. Thaumatin is today used mainly in desserts and chewing gums.
References
[67] J. H. Higginbotham in Alternative Sweeteners (Eds: L.O’Brien Nabors, R.C. Gelardi), Marcel Dekker, New York, USA, 1986. ISBN: 978-0-8247-7491-2
[68] H. van der Wel, K. Loeve, Eur. J. Biochem. 1972, 31, 221. DOI: 10.1111/j.1432-1033.1972.tb02522.x
[69] R. B. Iyengar et al., Eur. J. Biochem. 1979, 96, 193. DOI: 10.1111/j.1432-1033.1979.tb13029.x
[70] L. Edens et al., Gene 1982, 18, 1. DOI: 10.1016/0378-1119(82)90050-6
[71] A. M. de Vos et al., Proc. Natl. Acad. Sci. USA 1985, 82, 1406. Link
[72] R. Kaneko et al., Chem. Senses 2001, 26, 167. DOI: 10.1093/chemse/26.2.167
[73] T. Masuda et al., Acta Cryst. F 2011, 67, 652. DOI: 10.1107/S174430911101373X
[74] Protein Data Bank: 3ALD. DOI: 10.2210/pdb3ald/pdb
[75] Protein Data Bank: 3AOK. DOI: 10.2210/pdb3aok/pdb
The article has been published in German as:
- Die Saccharin-Saga,
Klaus Roth, Erich Lück,
Chem. unserer Zeit 2011, 45, 406–423.
DOI: 10.1002/ciuz.201100574
and
- Kalorienfreie Süße aus Labor und Natur,
Klaus Roth, Erich Lück,
Chem. unserer Zeit 2012, 46, 168–192.
DOI: 10.1002/ciuz.201200587
and was translated by W. E. Russey.
The Saccharin Saga – Part 1
The invention of the first artificial sweetener and a lifetime battle for credit
The Saccharin Saga – Part 2
The early industrial production and organized smuggling of saccharin
The Saccharin Saga – Part 3
The health concerns associated with artificial sweeteners
The Saccharin Saga – Part 4
A glance back to ancient Rome, and the most hair-raising of all sweeteners
The Saccharin Saga – Part 5
What’s in your softdrink? – Introducing cyclamate
The Saccharin Saga – Part 6
Aspartame – a sweet dipeptide ester
The Saccharin Saga – Part 7
Acesulfame-K – another successful sweetening agent
The Saccharin Saga – Part 8
Thaumatin – a sweet protein with a licorice aftertaste
The Saccharin Saga – Part 9
Sucrose or Splenda turned into a low-calorie alternative to sucralose or saccharose
The Saccharin Saga – Part 10
Combining sweet cations and anions, and turning bitter compounds into sweeteners
The Saccharin Saga – Part 11
Intelligent synthetic strategies for low-calorie sweeteners
The Saccharin Saga – Part 12
Stevia plant extracts as low-calorie sweeteners
The Saccharin Saga – Part 13
Finding the best mixture of sweeteners to replicate the taste of real sugar
See all articles by Klaus Roth published in ChemistryViews Magazine

.jpg)