The rapidly growing human population has led to inevitable consequences: more food has been consumed than produced in the past decade, the origin of the so-called “global food crisis”. To solve this crisis, food production needs to be significantly enhanced, and more sustainable and efficient crop protection strategies need to be applied.
Herbicides play an important role in controlling and inhibiting the growth of unwanted plants. In 2011, herbicidal ionic liquids (HILs, organic salts with a melting point below 100 °C) have been identified, which show several beneficial properties compared to commercial herbicides: limited volatility, reduced dose per hectare, and specific physicochemical properties (for example, increased hydrophobicity, which enhances adherence to the plant).
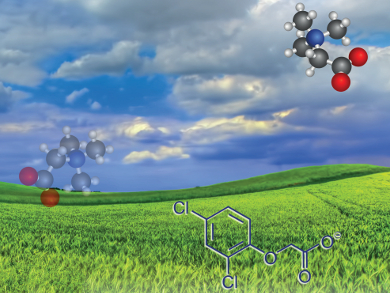
Juliusz Pernak, Poznan University of Technology, Poland, and colleagues synthesized several new herbicidal ionic liquids based on amino acid derivatives of natural origin (betaine and carnitine) as cations. Commercial herbicides such as 2,4-dichlorophenoxyacetic acid (2,4-D), 2-methyl-4-chlorophenoxyacetic acid (MCPA), methylchlorophenoxypropionic acid (MCPP), and 3,6-dichloro-2-methoxybenzoic acid (Dicamba) were used as anions. The new HILs show a low general toxicity combined with an enhanced herbicidal efficacy compared to commercial herbicides.
- Betaine and Carnitine Derivatives as Herbicidal Ionic Liquids,
Juliusz Pernak, Michał Niemczak, Łukasz Chrzanowski, Łukasz Ławniczak, Przemysław Fochtman, Katarzyna Marcinkowska, Tadeusz Praczyk,
Chem. Eur. J. 2016.
DOI: 10.1002/chem.201601952