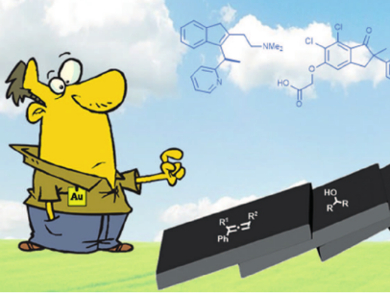
Highly substituted indenes are important motifs for biologically active compounds, in material science applications, and for homogeneous catalysis. Thus, synthetic approaches to these motifs are of interest. Some methods for the preparation of polysubstituted indenes have been reported, but a gold-catalyzed approach has not yet been applied.
Nuno Maulide and colleagues, University of Vienna, Austria, have developed a new synthetic route to highly substituted indenes in which an aryl-substituted allene reacts with an alcohol to undergo a domino C–C coupling sequence. This reaction forms two C–C bonds in inter- and intramolecular fashions, leading to the desired indenes in a single step, with water being the only side product. Using low catalyst loadings (1–2 mol% Au) and mild conditions (room temperature, under air), high yields of up to 96 % were obtained for the corresponding substituted indenes.
Notably, these reactions proceed without the need for an inert atmosphere or pre-drying of the reaction solvent. The aryl-substituted allenes could also be cyclized in the absence of the alcohol using gold catalysis. Preliminary mechanistic investigations suggest the involvement of carbocation intermediates.
- A Gold(I)-Catalyzed Domino Coupling of Alcohols with Allenes Enables the Synthesis of Highly Substituted Indenes,
Alexander Preinfalk, Antonio Misale, Nuno Maulide,
Chem. Eur. J. 2016.
DOI: 10.1002/chem.201603154