Tridecacyclene Accepts Electrons
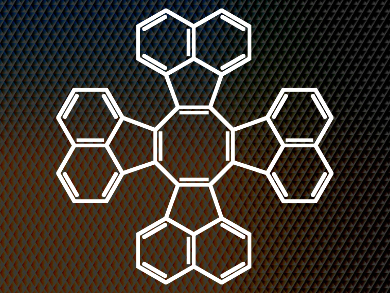
Tridecacyclene (pictured) is a tub-shaped polyaromatic hydrocarbon (PAH) and a wonderful example of this class of materials. In a sense, it traces an arc through chemical history back to German chemist Friedrich August Kekulé with his purported dream of Ouroboros, the serpent, biting its own tale and his revelation in the 1860s that benzene must be a six-membered ring. One might say that chemists have been fascinated by cyclic and ring systems ever since. The PAHs in all their forms are an important focus of modern chemistry.
Indeed, during the last three decades, the unique ability of PAHs to accept electrons reversibly without undergoing any kind of “shapeshifting” showed their great utility in electrochemistry and optoelectronics. PAHs with five-membered rings have been even more fruitful than those centered on a six-membered ring, according to Adam C. Whalley, University of Vermont, Burlington, USA, and colleagues.
Molecules with a Heart
The researchers point to corannulene, a PAH with a central five-membered ring, which is able to undergo reduction by accepting up to four electrons. This is due to its doubly degenerate lowest unoccupied molecular orbital (LUMO). In contrast, coronene, which is similar in structure but has a six-membered ring at its heart, can only accept two electrons.
The team points out that the range of PAHs has expanded over time and their number now includes structures with seven-membered and eight-membered rings at the center. This casts the net that might trap technologically useful materials even wider. As such, the team has turned to derivatives of 1,3,5,7-cyclooctatetraene (COT). This compound is an 8 π-electron carbocycle that adopts a tub-like structure in order to preclude the formation of a flat, but anti-aromatic eight-membered ring. The team explains how the Hückel definition of aromaticity would suggest that adding two electrons to this system would flatten this ring and make it aromatic. Indeed studies over the last few decades have suggested that this is what happens.
Upending a Molecular Tub
Evidence from the 1960s and the 1990s pointed to the likelihood that COT’s eight-membered ring would flatten upon the addition of two extra electrons, although this was never proven. Interestingly, variable-temperature nuclear magnetic resonance (NMR) spectroscopy performed on a substituted COT derivative has shown that this is likely not what is happening in this molecule at all. It does not form a stable, planar central ring, but rather it flips between two different tub conformations; endlessly turning itself inside out, one might say.
Moreover, crystal structures of some 47 metal complexes of COT did not clarify the situation. Instead, the structures seemed to suggest that it was the metal center that was pushing the relationship between planarity and aromaticity to its limits rather than there being anything intrinsically biased about the structure itself in the absence of the metal and any associated ligands.
Aromatic or Not?
The researchers recently devised a one-step synthesis of tridecacyclene, the cyclic tetramer of acenaphthylene. This molecule was confirmed to adopt the same tub shape as other COTs but with one important difference: the neighboring acenaphthylene units preclude the flattening of the middle eight-membered ring. As such, the team had hit on the perfect model, the ideal structure to help them define once and for all the relationship between planarity (or the lack thereof) and aromaticity in these compounds and perhaps their chemical cousins. Such an insight would facilitate the more rapid development of these materials into technologically useful organic units.
As such, the team produced the radical anion and dianion of tridecacyclene by potassium reduction of the parent compound. They then carried out a solid-state analysis of the resulting salts. The dipotassium salt of the dianion turned out to be aromatic around its central eight-membered ring, despite the fact that the overall three-dimensional structure of the compound, determined by X-ray diffraction, is tub-like and cannot be anything but. Fundamentally, this study demonstrates that aromaticity need not be tied to the planarity of a molecule, particularly with regard to COT derivatives.
“Our next step is to begin exploring the potential of these kinds of molecules for energy storage applications such as flow cell batteries,” Whalley told ChemViews Magazine.
- The reductive aromatization of tridecacyclene,
Daniel P Sumy, Aaron Finke, Adam Whalley,
Chem. Commun. 2016.
DOI: 10.1039/c6cc06078a
Also of Interest
- 150th Anniversary: Kekulé’s Benzene Structure,
ChemViews Mag. 2015.
Kekulé’s groundbreaking idea was published in 1865