Gold nanoparticles (NPs) with specific emission and absorption properties can be used in medical applications, both for diagnostic and treatment purposes. The stabilizing ligands used to prepare gold NPs usually play an important role for their emissive properties. For example, fluorogens attached to the nanoparticles can cause aggregation-induced emission (AIE).
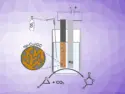
Kalyan K. Sadhu, Indian Institute of Technology Roorkee, Uttarakhand, India, and colleagues have developed an approach toward aggregation-induced emission in fluorogen-free gold nanoparticles. The team prepared gold nanoparticles via three different synthetic routes, using sodium citrate, sodium borohydride, and ethylenediaminetetraacetic acid (EDTA), respectively, as reducing agents. Diluted aqua regia (a mixture of nitric acid and hydrochloric acid which can dissolve gold and remove organic compounds) was then added to the NPs in a controlled manner.
The diluted acid causes the nanoparticles to aggregate, which induces near-infrared (NIR) luminescence. The luminescence was independent of the reducing agent used for the synthesis. The team performed preliminary tests on the biocompatibility of the aggregated NPs and found them to be nontoxic. The researchers were able to use the particles for imaging studies in human liver carcinoma cells.
- Fluorogen-free aggregation induced NIR emission from gold nanoparticles,
Meenaxi Saini, Yogeshwar Masirkar, Ritu Varshney, Partha Roy, Kalyan K. Sadhu,
Chem. Commun. 2017.
DOI: 10.1039/c7cc00641a