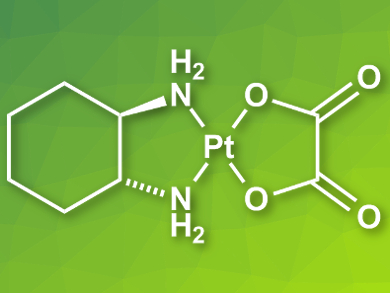
Platinum (II) complexes are commonly used as cancer drugs. Examples for such complexes are cisplatin [Pt(NH3)2Cl2], carboplatin [Pt(NH3)2(CBDCA)], and oxaliplatin [Pt(DACH)(Ox)] (pictured, CBDCA = 1,1-cyclobutanedicarboxylate, DACH = (1R,2R)-1,2-diaminocyclohexane, Ox = oxalate). These drugs are usually studied in aqueous solution, often with added dimethyl sulfoxide (DMSO). However, a decrease in anticancer activity has been observed for Pt complexes in the presence of DMSO.
Paul J. Dyson, Ecole Polytechnique Fédérale de Lausanne (EPFL), Switzerland, Hristo P. Varbanov, EPFL and University of Vienna, Austria, and colleagues have investigated the effects of water and DMSO on oxaliplatin. The team used electrospray ionization mass spectrometry (ESI-MS) and 1H-NMR spectroscopy to monitor the stability of oxaliplatin in pure DMSO, in DMSO/water mixtures, and in pure water.
The team found that oxaliplatin is stable in pure DMSO. However, when water was added to solutions of oxaliplatin in DMSO, the complex reacted rapidly and formed DMSO adducts. According to the researchers, this is caused by DMSO binding to the metal center after water has caused the cleavage of oxalate-Pt bonds. The bound DMSO then prevents the reattachment of the oxalate ligand. In pure water, the same bond cleavage happens, but the reattachment reaction is extremely fast, which causes the complex to be stable overall. The team states that DMSO should be avoided in biological experiments on Pt(II) complexes, since the water in biological systems could affect the results.
- Oxaliplatin reacts with DMSO only in the presence of water,
Hristo P. Varbanov, Daniel Ortiz, Doris Höfer, Laure Menin, Markus Galanski, Bernhard K. Keppler, Paul J. Dyson,
Dalton Trans. 2017.
DOI: 10.1039/c7dt01628j