Cycloisomerization reactions have been studied extensively because of their efficiency in building complexed structure from simpler acyclic compounds. The gold(I) catalyzed cycloisomerization of 1,5-enynes has been reported over a decade ago and studied by many groups. However, a highly enantioselective variant still needs to be developed. The challenge of this reaction is that both enantiomers of the 1,5-enyne can transform to either enantiomer of the bicyclo[3.1.0]hexene.
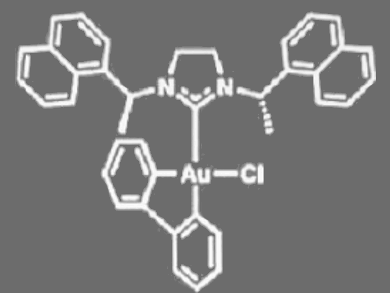
Patrick T. Bohan and F. Dean Toste, University of California, Berkeley, USA, have found that a gold(III) complex bearing a chiral N-heterocyclic carbene (see pictured) catalyzes enantioconvergent 1,5-enyne cycloisomerization. They also found that the product enantioselectivity decreases with increased conversion. Therefore, they developed an enantioconvergent kinetic resolution reaction to obtain both enantioenriched 1,5-enynes and bicyclo[3.1.0]hexene products with an s factor up to 48. The reactions were carried out in chloroform at –40 °C in the presence of AgBF4.
This transformation represents a rare mode of direct enantioconvergent kinetic resolution and is the first highly enantioselective reactions catalyzed by a well-defined gold(III) complex.
- Well-Defined Chiral Gold(III) Complex Catalyzed Direct Enantioconvergent Kinetic Resolution of 1,5-Enynes,
Patrick T. Bohan, F. Dean Toste,
J. Am. Chem. Soc. 2017, 139 (32), 11016–11019.
https:doi.org/10.1021/jacs.7b06025