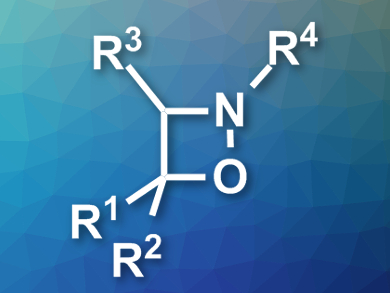
Four-membered heterocyclic compounds are useful synthetic intermediates in organic chemistry due to their high reactivity. However, for the same reason, methods for their preparation are sparse and often limited in substrate scope.
Weibing Liu and colleagues, Guangdong University of Petrochemical Technology, Maoming, China, have developed the first regioselective synthesis of polysubstituted 1,2-oxazetidines (pictured). The team used a [2 + 1 + 1] cycloaddition to combine styrenes and arylamines with tert-butyl hydroperoxide (TBHP), which acts both as an oxidant and as the oxygen source for the product. The reaction was performed in dioxane at 70 °C.
The researchers used the developed approach to prepare a broad range of substituted oxazetidines in good yields and with high regioselectivities. The reaction yield is dependent on the electronic nature of substituents on the styrenes: Electron-donating groups lower the yield, while electron-withdrawing substituents have a positive impact.
- Preparation of 1,2-Oxazetidines from Styrenes and Arylamines via a Peroxide-Mediated [2 + 1 + 1] Cycloaddition Reaction,
Weibing Liu, Cui Chen, Peng Zhou, Hua Tan,
Org. Lett. 2017.
DOI: 10.1021/acs.orglett.7b02796