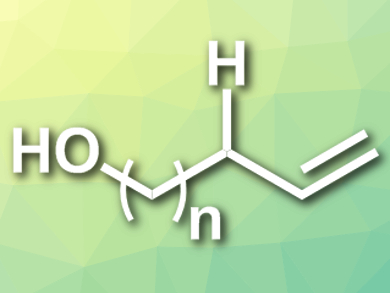
Alkenes are important in organic synthesis and are usually synthesized by prefunctionalization at the desired reaction site. The direct desaturation of aliphatic chains is less explored because the inherent difficulty of activating the kinetically inert C(sp3)–H bonds. Although radical approaches have been developed recently, they suffer from the regioselectivity problem.
Vladimir Gevorgyan, University of Illinois at Chicago, USA, and colleagues have developed a general, efficient, and site-selective remote desaturation protocol to synthesize allylic and homoallylic alcohols (pictured) in moderate to excellent yields from aliphatic alcohols with removable Si-auxiliaries. The reactions are induced by visible light and catalyzed by Pd(OAc)2 with 1-diphenylphosphino-1′-(di-tert-butylphosphino)ferrocene as a ligand and Cs2CO3 as a base in benzene at room temperature.
The reaction requires no exogenous photosensitizers or external oxidants. The hydrogen atom transfer process at unactivated C–H sites is achieved via a hybrid Pd-radical mechanism. Using an iodomethylsilane moiety as the auxiliary enables the site-selectivity.
- General, Auxiliary-Enabled Photoinduced Pd-Catalyzed Remote Desaturation of Aliphatic Alcohols,
Marvin Parasram, Padon Chuentragool, Yang Wang, Yi Shi, Vladimir Gevorgyan,
J. Am. Chem. Soc. 2017.
DOI: 10.1021/jacs.7b08459