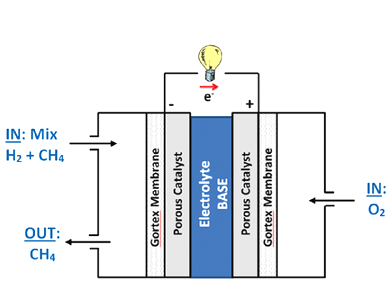
Klaudia Wagner, Prerna Tiwari, Gerhard F. Swiegers, and Gordon G. Wallace, University of Wollongong, Australia, have constructed alkaline fuel cells that efficiently use even very dilute hydrogen–methane mixtures as fuels. The fuel cells contain two porous, Gortex-based gas diffusion electrodes layered with Pd/Pt catalysts, binders, and a current carrying Ni mesh.
Mixtures of between 2 to 100 % of hydrogen were examined. Almost no difference between using pure hydrogen and 5 % hydrogen in methane, as the fuel was found. The Gortex substrates and the aqueous alkaline electrolyte are an active solid–liquid interface and ion conductor that allows the fuel cell to selectively extract the hydrogen from the methane and efficiently utilize it as a fuel. This system is much more efficient than the equivalent solid–solid catalyst interface and ion conductor in PEM fuel cells.
The cells were fully reversible after exposure to methane. This indicates that the methane gas has an inert behavior in the cell and that no catalyst deactivation occurs. According to the researchers, this type of fuel cells has the potential to harness the dilute gas mixtures of power-to-gas (P2G) for local generation of electrical power.
- Alkaline Fuel Cells with Novel Gortex-Based Electrodes are Powered Remarkably Efficiently by Methane Containing 5% Hydrogen,
Klaudia Wagner, Prerna Tiwari, Gerhard F. Swiegers, Gordon G. Wallace,
Adv. Energy Technol. 2017.
https://doi.org/10.1002/aenm.201702285