Heavier analogs of unsaturated organic molecules are often difficult to synthesize, since elements such as silicon or germanium generally do not form double bonds. Kinetic stabilization with large substituents can, however, allow the preparation of these interesting compounds. While a silicon analogue of cyclobutadiene had previously been synthesized, no free germanium analogue of cyclobutadiene without additional stabilization had been reported so far.
Tsukasa Matsuo, RIKEN Advanced Science Institute, Saitama, and Kindai University, Osaka, both Japan, and colleagues have synthesized the first example of such a germanium compound. The team used bulky EMind groups (EMind = 1,1,7,7-tetraethyl-3,3,5,5-tetramethyl-s-hydrindacen4-yl) to stabilize the four-membered germanium ring. They combined (EMind)2Ge: with GeCl2·dioxane to give the 1,2-dichlorodigermene (EMind)ClGe = GeCl(EMind). This intermediate was then converted to the cyclobutadiene germanium analogue Ge4(EMind)4 using lithium naphthalenide. The compound is air- and moisture-sensitive, but stable at room temperature under an argon atmosphere.
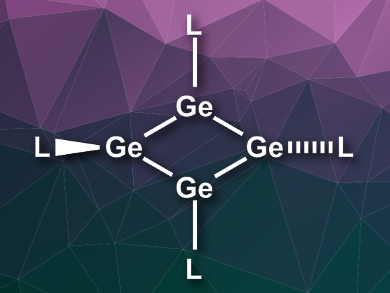
The compound was characterized using UV-Vis-NIR spectroscopy (NIR = near-infrared) and single-crystal X-ray diffraction. The molecule has a planar rhombic Ge4 ring, with two of the EMind substituents connected in-plane and two slightly out-of-plane. Density functional theory (DFT) calculations were used to better understand the bonding in the ring. According to the researchers, the ring features a 4π-electron antiaromaticity, which is stabilized by polar Jahn-Teller distortion.
- A stable free tetragermacyclobutadiene incorporating fused-ring bulky EMind groups,
Katsunori Suzuki, Yasuyuki Numata, Naoko Fujita, Naoki Hayakawa, Tomoharu Tanikawa, Daisuke Hashizume, Kohei Tamao, Hiroyuki Fueno, Kazuyoshi Tanaka, Tsukasa Matsuo,
Chem. Commun. 2018.
https://doi.org/10.1039/c7cc09443d