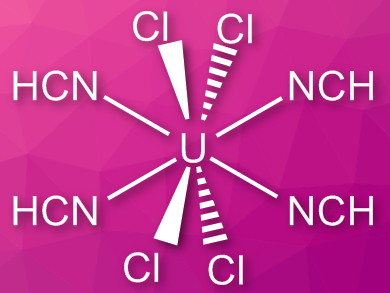
Coordination complexes of free hydrogen cyanide are quite rare, in contrast to cyanide complexes. This is partly due to the fact that the compound is toxic and hard to handle, with a boiling point close to room temperature.
Florian Kraus, University of Marburg, Germany, and colleagues have synthesized [UCl4(HCN)4], the first structurally characterized uranium(IV) complex with HCN ligands. The team sublimated anhydrous HCN onto powdered UCl4 and removed excess the HCN to give a moisture-sensitive, blue-green product.
The complex was characterized using powder X-ray diffraction, infrared (IR) spectroscopy, and powder neutron diffraction. When a vacuum is applied, HCN can be removed and UCl4 recovered. The complex decomposes at temperatures above 70 °C. According to the researchers, compounds such as [UCl4(HCN)4] could be useful for the chemical vapor deposition of nitride films or for the formation of uranium nitrides.
- [UCl4(HCN)4] – a hydrogen cyanide complex of uranium tetrachloride,
S. S. Rudel, C. Pietzonka, M. Hoelzel, F. Kraus,
Chem. Commun. 2018.
https://doi.org/10.1039/c7cc09401a