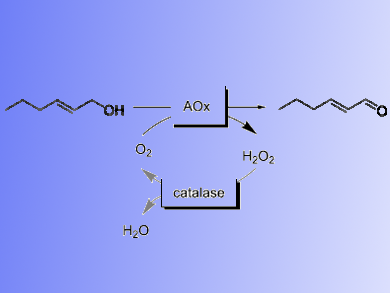
Trans-2-hexenal is a major component of the Green Notes aroma of fruits and vegetables. Green Notes aroma molecules are widely used in the flavor and fragrance industry as fresh flavor ingredients.
Frank Hollmann, Delft University of Technology, The Netherlands, and colleagues have developed a biocatalytic synthesis of trans-hex-2-enal from trans-hex-2-enol using a novel aryl alcohol oxidase from Pleurotus eryngii (PeAAOx). Oxidases use O2 as terminal electron acceptor for the oxidation reaction. The benefits of using O2 also come with the disadvantage
of its very poor solubility in aqueous media. Therefore, the team performed the reaction in a flow-reactor setup. It enables high O2 transfer rates while avoiding enzyme robustness issues frequently observed with ‘traditional’ aeration methods.
The team found high catalytic activities for the enzyme (turnover frequency up to 38 s−1 and turnover numbers more than 300,000). According to the researchers, their findings demonstrate the power of combining oxidase catalysis and flow chemistry.
- Biocatalytic synthesis of the Green Note trans-2-hexenal in a continuous-flow microreactor,
Morten M. C. H. van Schie, Tiago Pedroso de Almeida, Gabriele Laudadio, Florian Tieves, Elena Fernández-Fueyo, Timothy Noël, Isabel W. C. E. Arends, Frank Hollmann,
Beilstein J. Org. Chem. 2018.
https://doi.org/10.3762/bjoc.14.58
2.