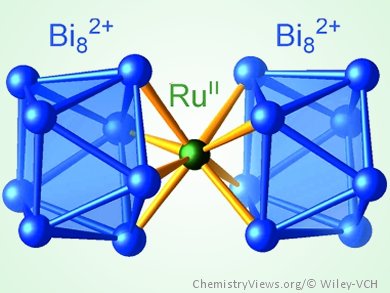
Bismuth is widely known for its broad range of oxidation states as found, for example, in its subvalent compounds, especially its polycations. In 1997, Bi24Ru3Br20, the first compound that contained bismuth polycations coordinated to a transition metal, was synthesized. As a consequence of a chemical ambiguity in this compound, the assignment of charges and, consequently, the interpretation of the chemical bonding in the clusters [Ru2Bi17Br4]m+ and [RuBi6Br12]n– remained an unresolved issue.
Michael Ruck, Technische Universität Dresden, Germany, and colleagues report a new bismuth–ruthenium–bromine system. Detailed investigations of the cluster compound [Ru2Bi14Br4](AlCl4)4 helped the team to gain a deeper insight into the coordination chemistry of bismuth polycations, as the charge of the cation could be precisely determined. By using this information, they were able to solve the structure of the heavily disordered compound Bi39.67(7)Ru2Br35.0(2). The elucidation of the heavily disordered structure together with quantum-chemical calculations and comparative analysis with structurally related compounds enabled a deeper insight into the coordination of bismuth polycations and polyanions, which, similar to arenes, can act as multihapto multielectron donor ligands.
- [Ru(Bi8)2]6+ – A Cluster in a Highly Disordered Crystal Structure is the Key to the Understanding of the Coordination Chemistry of Bismuth Polycations,
Matthias F. Groh, Anna Isaeva, Christoph Frey, Michael Ruck,
Z. anorg. allg. Chem. 2013, 639, 2401–2405,
DOI: 10.1002/zaac.201300377