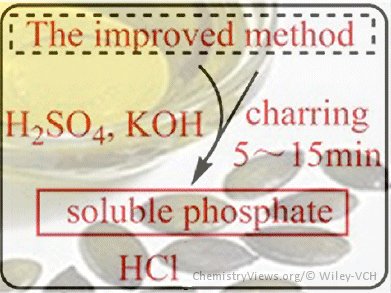
The phosphatide content of vegetable oils determines their quality and is a means to monitor the refining processes. The Official Method of the American Oil Chemists’ Society (AOCS), Ca 12–55, has gained wide acceptance for routine quality control owing to its simple set-up and low cost. The phosphorous content is determined by charring and ashing the oil sample with zinc oxide (ZnO), followed by the calorimetric measurement of phosphorus as blue phosphomolybdic acid. The drawbacks are the length of the charring–ashing process, unsafe operation owing to the easy splashing of the oil sample, and the formation of insoluble inorganic phosphate.
Rong Li, Northwest University, Xi’an, China, and colleagues improved the procedure of the AOCS Official Method Ca 12–55 by the addition of H2SO4 and KOH to the vegetable oil sample instead of ZnO. The addition of H2SO4 shortened the pretreatment process and by using KOH instead of ZnO only soluble phosphate was formed. Therefore, this approach bypasses the major drawbacks of the method, while it also does not sacrifice accuracy or precision and shows a good consistency to the official method. However, more experimental data for other vegetable oils are still required.
- An improved method for determining the phosphorus content in vegetable oils,
Bin Chen, Xia Xiao, Rong Li, Wanli Zhao, Kaidi Yang, Guoliang Chen, Xiaoxun Ma,
Europ. J. Lipid Sci. Technol. 2014.
DOI: 10.1002/ejlt.201300378