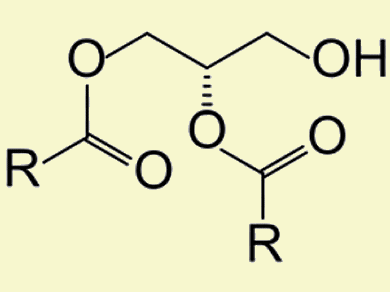
Lipases are enzymes that catalyze the esterification of fatty acids (FA) with glycerol. They are used in the production of monoacylglycerides (MAG) and diacylglycerides (DAG; pictured), owing to their substrate specificity with respect to MAG and DAG rather than triacylglycerides (TAG). Their application for the industrial scale synthesis of DAG-enriched oils strongly depends on the immobilization of the enzyme, as immobilized enzymes are often more robust than free enzymes, easy to recover, and can be reused in a convenient and continuous operation.
Yonghua Wang, South China University of Technology, Guangzhou, China, and colleagues tested the enzyme activity of SMG1, a novel lipase, in the immobilized state. The lipase was linked to a resin (Resin D380) by ionic bonding between the functional NH2 groups and the amino acid residues of the lipase. A high degree of esterification of 68 % was obtained and the immobilized lipase retained 49.7 % of its initial activity after being used for six times. The immobilized SMG1 also exhibits higher temperature tolerance of up to 40 °C compared to the free lipase, which could only tolerate temperatures up to 30 °C.
All these features should promote the application of enzymes in the oils and fats industry.
- Immobilization of lipase SMG1 and its application in synthesis of partial glycerides,
Weifei Wang, Yang Xu, Xiaoli Qin, Dongming Lan, Bo Yang, Yonghua Wang,
Eur. J. Lipid Sci. Technol. 2014.
DOI: 10.1002/ejlt.201300442