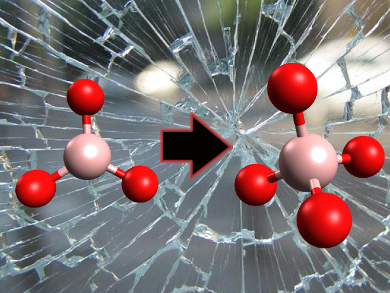
Additives such as boron oxide are commonly used to optimize the properties of glass, including chemical durability, flow resistance, optical transparency, and thermal expansion. In borosilicate glass the structure around the boron atoms changes with pressure and temperature. It switches from planar BO3 to a BO4 tetrahedron. Until now, it was impossible to study the transition of these structures.
Sabyasachi Sen, University of California (UC) Davis, USA, and colleagues have used in situ high-pressure (up to 2 GPa) boron-11 solid-state nuclear magnetic resonance (NMR) spectroscopy in combination with ab initio calculations to capture atoms in borosilicate glass while it flips from one structure to another
First the planar BO3 deforms into a pyramid shape, with the boron atom pushed up. That may bring it close to another oxygen atom, and let the structure turn into a BO4 tetrahedron.
Intriguingly, although glass is structurally isotropic and the stress on the glass is the same in all directions, the boron atoms respond by moving in one direction in relation to the rest of the structure.
These finding may help understand stress-induced phenomena in amorphous materials.
- Observation of the transition state for pressure-induced BO3→ BO4 conversion in glass,
Trenton Edwards, Takatsugu Endo, Jeffrey H. Walton, Sabyasachi Sen,
Science 2014.
DOI: 10.1126/science.1256224