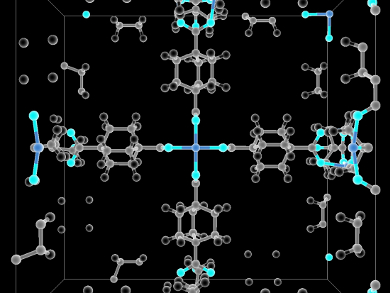
While natural gas is an efficient fuel for cars, storing it in a relatively compact vehicle is a challenge. High-pressure, low-temperature methane tanks are bulky, limiting the use in automobiles. A possible alternative is adsorbing CH4 to metal-organic frameworks (MOFs), cage-like structures composed of metal ions and organic linkers.
Michael W. Deem, Rice University, Houston, TX, USA, and colleagues have developed a computational methodology that allows optimizing MOFs for methane storage, yet limits its search to linkers that can be readily synthesized from common precursors. Using Monte Carlo simulations, the study found 48 MOFs that are predicted to beat the best currently available capacity by as much as 8 %.
This result is a step toward the creation of MOFs with designed properties and should enable targeted experiments to find improved materials for methane storage.
- In Silico Discovery of High Deliverable Capacity Metal-Organic Frameworks,
Yi Bao, Richard Luis Martin, Cory M Simon, Maciej Haranczyk, Berend Smit, Michael W Deem,
J. Phys. Chem. C 2014.
DOI: 10.1021/jp5123486