Gas absorption in single-layer falling films has been intensely studied. However, the mass transfer in wavy two-layer films has not been modeled so far. The main difficulty in computing mass transfer is the complexity of solving the hydrodynamical problem to find finite-amplitude wave regimes.
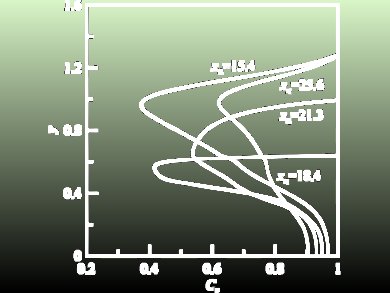
G. Çekiç and G. M. Sisoev, University of Birmingham, UK, have studied the absorption of a weakly soluble gas into a two-layer film flowing down a vertical wall. The analysis is based on an approximate long-wave model generalizing the Kapitza–Shkadov model for a single-layer film. The suggested algorithm for computing the gas absorption into a two-layer falling film contains two steps: (i) computing the hydrodynamical problem for the evolution equations and (ii) solving the diffusion problem. Reducing the full Navier–Stokes problem to the evolution equations is a condiderable improvement, and saves computational resources in finding optimized regimes of absorption. The algorithm can be generalized for other types of two-layer flow, for example flows over a spinning disk.
It was found that wavy regimes in the film strongly affect the absorption rate. Similar to single-film flow, the absorption rate is increased for wavy regimes in comparison with the waveless flow. This amplification takes place at the interface between the liquids as well as at the film surface. Numerous transient computations have demonstrated that the growth of the absorption rate is explained by the nonuniform distribution of the concentration gradient along the film surface and the interface. The largest gradients and thus largest local gas fluxes at the surface and interface are observed at wave troughs, where the thickness of the first layer and the whole film are minimal.
- Gas absorption into a wavy two-layer falling film,
G. Çekiç, G. M. Sisoev,
AIChE J. 2015.
DOI: 10.1002/aic.14778