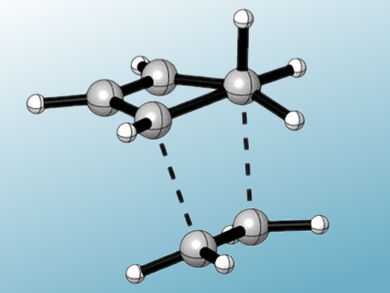
The pervasive influence of aromaticity was explained by the late Paul von Ragué Schleyer though hyperconjugation. Hyperconjugation – the donation of electrons in a σ-orbital to an adjacent π-orbital – stabilizes aromaticity, while negative hyperconjugation leads to antiaromaticity.
In a special issue of the Journal of Computational Chemistry paying tribute to Professor Schleyer, Kendall Houk and colleagues model a Diels-Alder reaction to show that hyperconjugation plays a role in determining reactivity. The rate of Diels-Alder reactions involving 5-substituted cyclopentadienes relies heavily on the substituent at the 5-position. Depending on the functional group, over a billionfold variation in relative reaction rates is seen. To understand this surprising effect, the team modeled the transition structures of reactions involving various substituents using density functional theory.
They found that the geometry of the substituted cyclopentadienes is distorted by hyperconjugative interactions between the diene and the substituent at the 5-position. When hyperconjugation is present, the interaction is stabilizing. A stabilized geometry requires more energy to distort to the transition state geometry, thus increasing the activation energy and reducing reactivity. Negative hyperconjugation leads to destabilization of the initial complex, which decreases the activation energy, allowing the reaction to proceed more readily.
The result is that Professor Schleyer’s notion of hyperconjugation explains the large substituent effect on the reactivity of 5-substituted cyclopentadienes in the Diels-Alder reaction. In future studies, the researchers plan to examine the role of geometric distortions on the π-facial stereoselectivity of the Diels-Alder reaction.
- Schleyer hyperconjugative aromaticity and Diels-Alder reactivity of 5-substituted cyclopentadienes,
Brian J. Levandowski, Lufeng Zou, K. N. Houk,
J. Comput. Chem. 2015.
DOI: 10.1002/jcc.24191