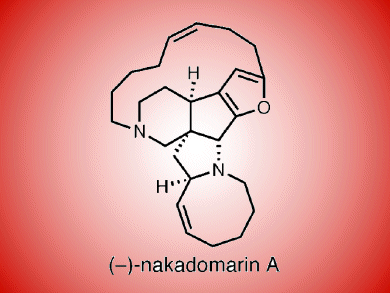
One of the most fascinating manzamine natural products is nakadomarin A, which was isolated by Kobayashi in 1997 from an Okinawan sponge Amphimedon sp. It has a complex 6/5/5/5/8/15 hexacyclic ring system and embodies a furan ring.
Kobayashi suggested a biosynthetic route to the structure, but now a concise synthesis that includes an enecarbamate Michael addition/furan-N-acyliminium ion cascade cyclization leads to the tetracyclic core. Mark Nilson and Raymond Funk, Pennsylvania State University, USA, then employed a ring-closing alkyne and alkene metatheses which allowed them to construct the fifteen- and eight-membered azacycles, respectively.
- Total Synthesis of (−)-Nakadomarin A
M. G. Nilson, R. L. Funk,
Org. Lett. 2010, 12.
DOI: 10.1021/ol102079z - For an overview of recent attempts to make (−)-Nakadomarin A, see:
Concise Synthesis of (−)-Nakadomarin A
D. B. C. Martin, C. D. Vanderwal,
Angew. Chem. Int. Ed. 2010, 49, 2830–2832.
DOI: 10.1002/anie.201000045