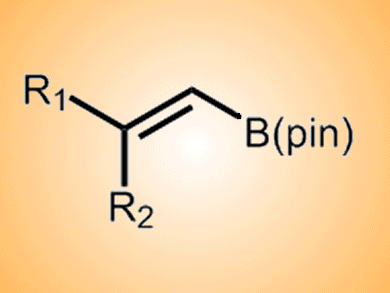
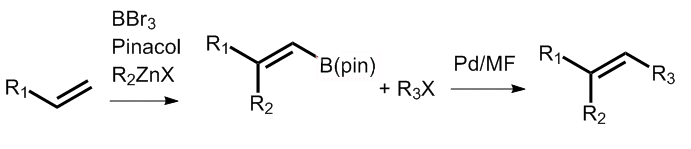
The Negishi–Suzuki tandem alkenylation makes use of boron’s high electronegativity to give a selective route to trisubstituted alkenes. However, in some cases, notably propyne haloboration, selectivity is ≤ 90 %.
Ei-ichi Negishi and colleagues, Purdue University, USA, report a Pd-catalyzed alkenylation that employs fluoride and alkenyl(pinacol)boranes to give trisubstituted alkenes in good to excellent yields with over 98 % stereoselectivity.
Disubstituted-1-alkenyl(pinacol)boranes were prepared from propyne via propyne bromoboration—Pd-catalyzed Negishi alkenylation either in one pot or in two steps. The fluoride salts TBAF (95 %), Et4NF (87 %), and CsF (90 %) were found to be the most effective for promoting the reaction of the disubstituted alkenylboranes with p-nitroiodobenzene in the presence of a Pd catalyst to give the methyl-branched (Z)-trisubstituted alkenes in high isomeric purity.
- Highly (98 %) Selective Trisubstituted Alkene Synthesis of Wide Applicability via Fluoride-Promoted Pd-Catalyzed Cross-Coupling of Alkenylboranes
E.-i. Negishi, T. Tobrman, H. Rao, S. Xu, C.-T. Lee,
Isr. J. Chem. 2010, 50.
DOI: 10.1002/ijch.201000051