Arylboronates are important intermediates in organic synthesis. The traditional way to synthesize these compounds involves aryllithium or Grignard reagents and organoboron nucleophiles. However, these processes require multiple steps and have poor functional group tolerance. Transition-metal catalysis can circumvent these problems, but often requires expensive noble metals such as palladium.
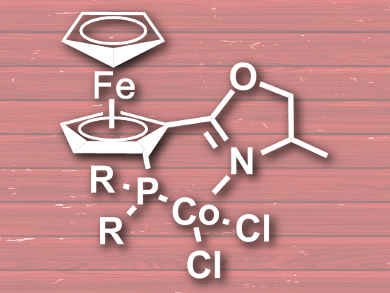
Aiguo Hu, East China University of Science and Technology, Shanghai, Zheng Huang, Shanghai Institute of Organic Chemistry, China, and colleagues have developed a cost-effective cobalt-catalyzed borylation of aryl halides and aryl pseudohalides. The catalyst is a ferrocenyloxazoline derivative (pictured), and was synthesized by addition of diphenylphosphine or diisopropylphosphine to deprotonated ferrocenyloxazoline and a subsequent reaction with CoCl2.
The researchers tested the catalyst’s efficiency in reactions of aryl halides, aryl triflates, aryl mesylates, and aryl diazonium salts with bis(pinacolato)diboron. They found that the cobalt compound was able to catalyze the borylations under moderate conditions (at 50 °C over 24 hours) and for a wide scope of substrates. The reactions proceeded in moderate to good yields and with good functional group tolerance.
- Cobalt-Catalyzed Borylation of Aryl Halides and Pseudohalides,
Wubing Yao, Huaquan Fang, Sihan Peng, Huanan Wen, Lei Zhang, Aiguo Hu, Zheng Huang,
Organometallics 2016.
DOI: 10.1021/acs.organomet.6b00161