Direct alcohol fuel cells (DAFCs), such as those using methanol, are cheaper and more portable than hydrogen-based fuel cells, making them useful for a range of applications. However, their efficiency is usually considerably lower. Polyalcohols such as ethylene glycol or glycerol have higher energy densities than methanol or ethanol and are thus an interesting alternative fuel. The development of polyalcohol fuel cells is hindered by the limited stability and activity of the available nanocatalysts for polyalcohol electrooxidation.
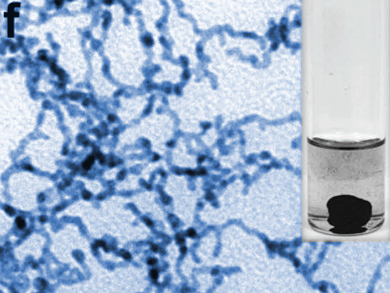
Xiaoqing Huang, Soochow University, Jiangsu, China, and colleagues have developed 3D-networked platinum-lead nanowires (pictured) as a catalyst for the ethylene glycol oxidation reaction (EGOR). The team used a simple wet-chemical approach and combined K2PtCl4 as a platinum source, Pb(acac)2 (acac = diacetylacetonate) as a lead source, citric acid as a reducing agent, polyvinylpyrrolidone (PVP) as a capping agent, and ethylene glycol (EG) as both solvent and reducing agent. The composition of the resulting Pt/Pb nanowires could be tuned by simply changing the reaction temperature.
The researchers tested the catalyst in the ethylene glycol oxidation reaction and found that the material outperforms both commercial Pt/C and pure networked platinum nanowires. The researchers attribute this to the nanowires’ high surface area and their large number of catalytically active defect sites, as well as to the fact that the lead component prevents catalyst poisoning.
- 3D Platinum–Lead Nanowire Networks as Highly Efficient Ethylene Glycol Oxidation Electrocatalysts,
Yonggang Feng, Lingzheng Bu, Shaojun Guo, Jun Guo, Xiaoqing Huang,
Small 2016.
DOI: 10.1002/smll.201601620