Compounds with zero-valent transition metals (metals atoms in the oxidation state 0) are common, e.g., in transition metal catalysis. In contrast, zero-valent main group species have only recently become a topic of interest. Such E(0) species have been found, e.g., for the group 14 elements silicon, germanium, and tin, usually stabilized by bulky donating ligands such as N-heterocyclic carbenes (NHCs). In such complexes, the group 14 element is sterically protected, which can limit its reactivity.
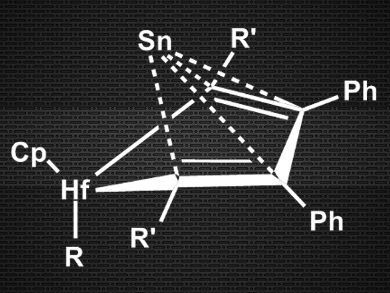
Masaichi Saito, Saitama University, Japan, and colleagues have synthesized a new type of tin(0) compound, where the Sn(0) atom is much less sterically shielded than in NHC complexes. The team reacted a dilithiostannole with [Cp2HfCl2] (Cp = cyclopentadienyl) in tetrahydrofuran (THF) and obtained (η4‑butadiene)Sn(0) complexes (pictured).
The researchers characterized the products using X-ray crystallography and 1H, 13C, and 119Sn NMR spectroscopy. They were able to confirm the pyramidal structure and the η4-type bonding between butadiene and tin. According to the team, this makes the compounds the first examples of butadiene complexes with group 14 metals. Density functional theory (DFT) calculations corroborate the Sn(0) character of the tin atom. This new type of zero-valent main group compound could exhibit previously unexplored reactivity.
- (η4-Butadiene)Sn(0) Complexes: A New Approach for Zero-Valent p-Block Elements Utilizing a Butadiene as a 4π-Electron Donor,
Takuya Kuwabara, Marisa Nakada, Jumpei Hamada, Jing Dong Guo, Shigeru Nagase, Masaichi Saito,
J. Am. Chem. Soc. 2016.
DOI: 10.1021/jacs.6b07304