Amines are an important class of products in organic synthesis. One common method to prepare amines is the hydrosilylation of nitriles, amides, imines, etc. However, the reduction of imines in this manner usually requires expensive noble metal catalysts.
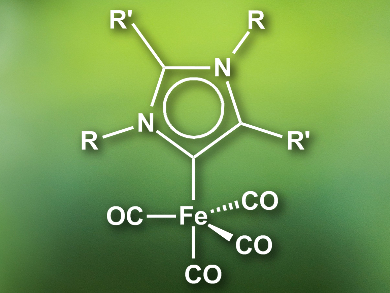
Swadhin K. Mandal, Indian Institute of Science Education and Research, Kolkata, India, and colleagues have developed an iron(0) catalyst for the hydrosilylation of imines to obtain amines. In contrast to the metals used so far, iron is inexpensive and environmentally benign. The catalyst (pictured) features an abnormal N-heterocyclic carbene (aNHC) ligand. aNHCs are bound to the metal at the C4 or C5 position, in contrast to C2 for “normal” NHCs. Abnormal NHCs are better σ-donors and increase the electron density at the catalyst’s iron center compared with normal NHCs. The improves the catalytic activity.
The researchers prepared the catalyst by combining the aNHC with diiron nonacarbonyl, [Fe2(CO)9], in toluene at room temperature. The efficiency of the catalyst was tested in the hydrosilylation of various aldimines and ketimines. The system has high turnover numbers up to 17,000 and was able to efficiently catalyze the reactions at room temperature, with good functional group tolerance, and good chemoselectivity. Remarkably, the catalytic activity of the iron(0) complex is higher than that of reported noble-metal catalysts.
- A Highly Efficient Base-Metal Catalyst: Chemoselective Reduction of Imines to Amines Using An Abnormal-NHC–Fe(0) Complex,
Mrinal Bhunia, Pradip Kumar Hota, Gonela Vijaykumar, Debashis Adhikari, Swadhin K. Mandal,
Organometallics 2016.
DOI: 10.1021/acs.organomet.6b00478