The production of lithium is projected to increase by 230 % from 2010 to 2020 as the demand for lithium ion batteries increases. The majority of Lithium worldwide production comes from brines. Brines contain Li+ but also other dissolved salts such as Ca2+, Mg2+, and borates.
Currently, purification techniques use liquid-liquid extraction, solvents, and filtration to remove BO3/BO4–, Ca2+, and Mg2+. Mg2+ is particularly challenging to remove because Mg2+ and Li+ have similar properties in many solvents, including water, which complicates purification processes such as liquid-liquid extraction procedures.
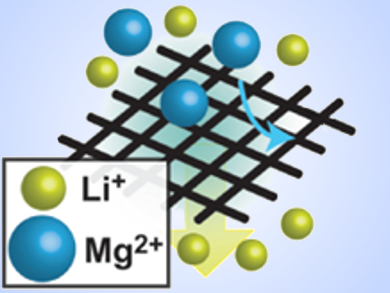
Hang-Ah Park and colleagues, Carnegie Mellon University, Pittsburgh, PA, USA, used redox-active catechol-bearing polydopamine melanins to coat stainless steel meshes. The catechols selectively chelate Mg2+ ions and separate them from Li+ ions in aqueous solutions at concentrations that are comparable to those of the brines used for sourcing raw lithium.
According to the researchers, catechol-based chelation of divalent cations can aid in purifying Li+ from brines in a cost-effective, chemically stable, and scalable manner.
- Lithium purification from aqueous solutions using bioinspired redox-active melanin membranes,
Hang-Ah Park, Young Jo Kim, Ik Soo Kwon, Luke Klosterman, Christopher J Bettinger,
Polym. Int. 2016, 65, 1331–1338.
DOI: 10.1002/pi.5184The article was published in the Special Issue: Melanin in Polymer International
Also of Interest
- Industry Roundup: Resources for Emerging Technologies,
ChemViews Mag. 2016.
DOI: 10.1002/chemv.201600079
— New technologies will shape the demand for raw materials