
Largest Pilot Plant for Bioaerogels Production
The new plant at the Technical University of Hamburg-Harburg, Germany, will be used to explore ways to reduce the overall cost of industrial production of aerogels
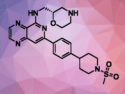
Selective Inhibitor of Spleen Tyrosine Kinase Discovered
Drug against autoimmune diseases and cancers in development
![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)
Calix[4]arene “Handshakes” via Urea–Carboxylate interactions
Supramolecular capsules that can be reversibly disassembled

Bio-Sourced Hydroxyurethanes
Regioselective synthesis of carbamates from bioderived cyclic carbonates and amines