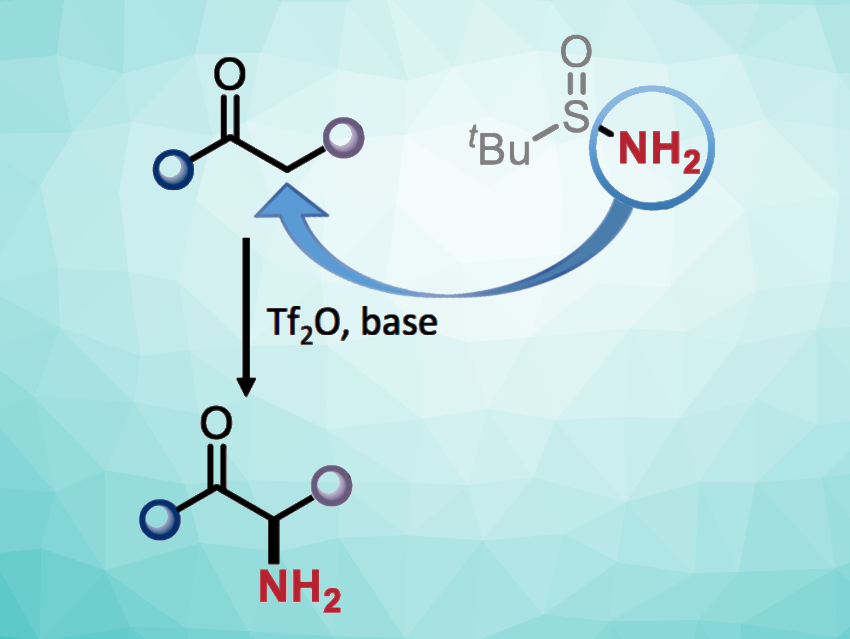
The ability to introduce an amino group into a small molecule at a predetermined position is important in synthetic chemistry. The installation of free primary amino groups can be particularly useful, but only a few methods exist for the preparation of primary α-amino carbonyls, for example. In many cases, this type of amination can require the prefunctionalization of the carbonyl substrate.
Nuno Maulide, University of Vienna, and Christian-Doppler Laboratory for Entropy-Oriented Drug Design, Vienna, Austria, and colleagues have developed a method for the straightforward transfer of a free amino group from a commercially available nitrogen source to unfunctionalized carbonyl compounds (amides and ketones), i.e., a direct α-amination (general reaction pictured). The team used tert-butanesulfinamide (tBuS(O)NH2) as the nitrogen source for the α-amination of a variety of amides and different electron-rich aryl ketones in the presence of trifluoromethanesulfonic anhydride (Tf2O) and 2-iodopyridine, using CH2Cl2 as the solvent. The reactions were performed at 0 °C to 20 °C.
The desired primary amine products were obtained in moderate to good yields. They can be further transformed, e.g., via peptide coupling in a one-pot manner. The team proposes a reaction mechanism that involves a nucleophilic attack of tert-butanesulfinamide on, e.g., a keteniminium intermediate that is generated from an amide, giving a sulfonium species. This is followed by a [2,3]-rearrangement to give a sulfenamide. The desired product is then obtained via an acidic workup. According to the researchers, the work could be useful, e.g., for the synthesis of unnatural α-amino acid derivatives.
- Free Amino Group Transfer via α‐Amination of Native Carbonyls,
Minghao Feng, Anthony J. Fernandes, Ana Sirvent, Eleonora Spinozzi, Saad Shaaban, Nuno Maulide,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202304990