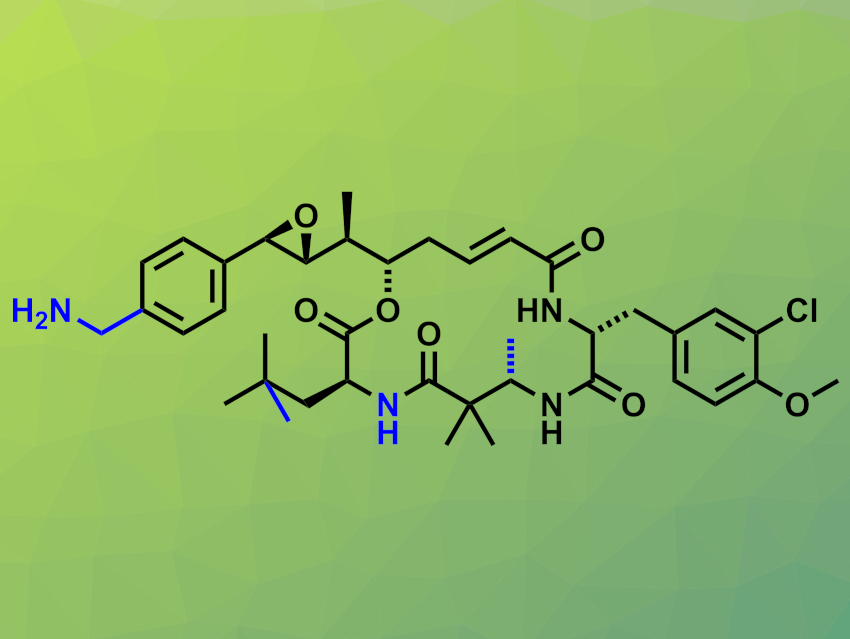
Cryptophycins are potent cytotoxic macrolides containing 16-membered rings. They were isolated from blue-green algae. This family of compounds has shown promising antitumor activities. More stable and less toxic derivatives could be useful candidates for anticancer drug development, in particular, as payloads in antibody–drug conjugates. This requires synthesizing derivatives with suitable functional groups for attachment to an antibody.
For example, a p-(aminomethyl)phenyl aza-cryptophycin analogue (pictured) has been developed as a candidate for antibody attachment. It has been modified in several places (marked in blue) compared with the cryptophycin parent compound: The p-(aminomethyl)phenyl group allows for covalent attachment to an antibody, an ester linkage was replaced with an amide, and methyl groups were added. However, the synthesis of this analogue requires 27 steps and provides only low yields of about 0.6 %.
Antony Bigot, Sanofi, Vitry-sur-Seine, France, and colleagues have developed a shorter synthesis of this aza-cryptophycin analogue with much higher yields. The team used esterification and amidification reactions to connect the compound’s building blocks, a trans-selective ring-closing metathesis reaction to form the 16-membered ring, and an asymmetric Corey–Chaykovsky-type reaction to create the epoxide group.
The new synthesis is shorter (19 steps) and more efficient (16 % yield for the longest linear sequence) than the previously developed approach. It can be performed on a gram scale and provides sufficient material for further studies.
- Fit-for-Purpose Synthesis of an Aza-Cryptophycin Analogue as the Payload for an Antibody–Drug Conjugate,
Audrey Bendelac, Françoise Benedetti, Valerie Doublet-Decabras, Rachel Lokovi, François Decalogne, Antony Bigot,
Org. Process Res. Dev. 2022.
https://doi.org/10.1021/acs.oprd.2c00080