In homogeneous catalysis, the catalysts remain in the same phase as the reagents. This means that products are usually extracted from the reaction mixture, e.g., by distillation or other means of phase separation, and the recovery and recycling of the catalyst can be difficult.
Xiao Su, University of Illinois Urbana-Champaign, Urbana, USA, and colleagues have found an electrosorptive solution to the recycling problem—by devising an electrochemical recycling scheme to extract soluble catalysts directly from the reaction mixture. While the product solution leaves the reactor cell, the metal-based catalyst is adsorbed on the electrode material and recycled in the next reactant flow.
Redox-Active Electrodes
The recycling electrode contains a redox-active coating, which switches to an adsorbing state when a voltage is applied. The soluble catalyst then binds to the adsorption sites, until the electrode is completely covered. When the electrode potential is set to zero or negative values, the catalyst is released.
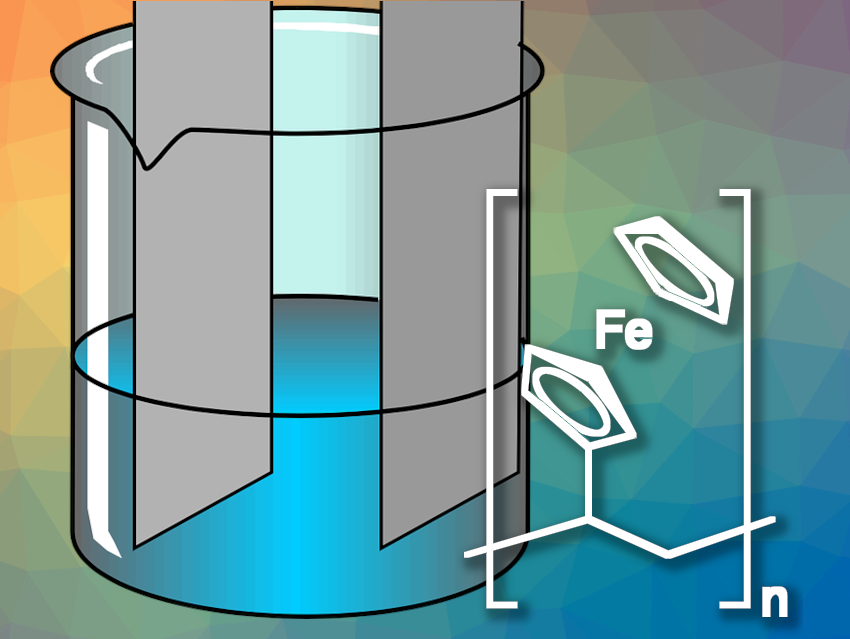
To redox-functionalize the electrode, the researchers used poly(vinylferrocene) (pictured), the polymerization product of vinylferrocene. Poly(vinylferrocene) includes the ferrocene/ferrocenium cation redox couple, which has its transition at low positive potentials and binds anionic metal complexes. The team also provided the polymer with a porous support made of carbon nanotubes, applied to the electrode as a coating ink.
The researchers chose industrially relevant catalysts from the platinum group to prove the feasibility and relevance of their electrosorption concept. Despite their high price and the difficulty recycling them, platinum-group catalysts promote some of the most important chemical processes. For example, chloroplatinum derivatives have been the driving force behind silicone chemistry since the 1950s, when chloroplatinic acid was found to promote hydrosilylation. In addition, organopalladium catalysts are used in a variety of commercial applications, such as Wacker oxidations, the oxidation of ethylene to acetaldehyde, and in Suzuki and related cross-coupling reactions to form carbon–carbon bonds.
The active form of these catalysts is a soluble complex which cannot be extracted from the reaction simply by filtration, meaning it is the product that has to be separated off. Some products simply leave the reactor in the form of a gas, while in hydrosilylation, the products are distilled.
However, distillation is energy-intensive and costly. The concept introduced by the team can recycle the catalyst, works at room temperature, and can be run in a flow-cell setup under mild conditions.
Electrochemical Flow-Cell Design
The team devised a flow-cell reaction scheme with a reactor and an electrochemical cell placed either upstream or downstream of the reactor. When the electrochemical cell is downstream of the reactor, a positive voltage is applied to remove the catalyst from the product stream. If it is upstream of the reactor, the catalyst can be recycled back into a fresh stream of reactants by applying zero, or a slightly negative, voltage. The team also set up a continuous operation mode by placing the electrodes directly in the reactor for immediate catalyst extraction from the products.
The team reported an uptake of up to 200 mg of platinum per gram of adsorbent. The catalyst also remained active across several cycles. The system tolerated a variety of electrolytes, based on, e.g., water, ethanol, acetone, and tetrahydrofuran (THF), and was not influenced by salts often added in industrial settings to stabilize the catalysts.
Looking in more detail at the mode of binding, the researchers observed that the main interactions took place between the ligands and the cyclopentadiene ring, either from negative chlorides or from phenylphosphane ligands in the case of Suzuki catalysts. The team envisions that electrosorption under mild conditions could also be applicable to other homogeneous catalysts, providing more efficient chemical manufacturing and significant savings on resources.
- Electrochemical recycling of homogeneous catalysts,
Stephen Cotty, Jemin Jeon, Johannes Elbert, Vijaya Sundar Jeyaraj, Alexander V. Mironenko, Xiao Su,
Sci. Adv. 2022.
https://doi.org/10.1126/sciadv.ade3094