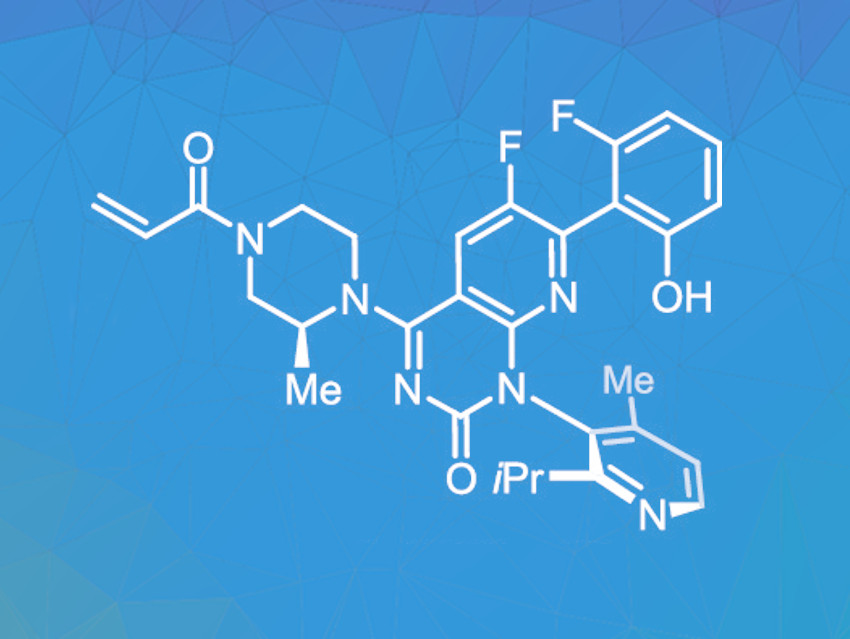
The protein KRAS usually regulates the growth and differentiation of cells. If the gene coding for this protein mutates, uncontrolled cell growth and malignant tumors can be the consequence. Sotorasib (pictured) can inhibit the growth of such tumor cells. It has been approved for the treatment of non-small-cell lung cancers, and further clinical studies for other types of cancer are underway. The growing demand for this drug requires an efficient commercial synthesis route that can provide the product in large quantities at acceptable costs.
Andrew T. Parsons, Amgen, Inc., Cambridge, MA, USA, and colleagues have developed a commercial process for manufacturing sotorasib based on the route originally used for the synthesis of the drug. The first synthesis step involves a chlorination of the starting material by phosphoryl chloride, followed by an amination. Initially, the yield of this reaction was relatively low because a dimeric side product was formed during the chlorination. This undesired reaction was prevented by changing the order and speed of reagent addition, the temperature, and the concentration of phosphoryl chloride. More efficient work-up further increased the yield from 66 % to 82 %.
Next, a palladium-catalyzed coupling reaction was carried out. For this step, the solvent was changed from dioxane to the more environmentally benign 2-methyltetrahydrofuran, and the palladium loading was lowered considerably by using a more active catalyst. Afterward, a Boc protecting group was removed using trifluoroacetic acid. This step was improved by optimizing the solvent combination. Then the acryl amide group was introduced using acryloyl chloride, completing the formation of the molecular structure. Here, the addition of trifluoracetic acid was found to prevent the formation of impurities.
Finally, the raw product was recrystallized from a mixture of ethanol and water to remove any remaining impurities. The desired crystal form of the final drug was obtained with over 99.5 % purity as determined by high-performance liquid chromatography (HPLC).
This improved synthesis route provides sotorasib in quantities of several hundred kilograms in high purities and with an overall yield of 65 % instead of the previously achieved 38 %. This process could prove access to the drug in significant amounts for more widespread use.
- Development of a Commercial Manufacturing Process for Sotorasib, a First-in-Class KRASG12C Inhibitor,
Liang Zhang, Daniel J. Griffin, Matthew G. Beaver, Laura E. Blue, Christopher J. Borths, Derek B. Brown, Seb Caille, Ying Chen, Alan H. Cherney, Brian M. Cochran, John T. Colyer, Michael T. Corbett, Tiffany L. Correll, Richard D. Crockett, Xi-Jie Dai, Peter K. Dornan, Robert P. Farrell, Simon J. Hedley, Hsiao-Wu Hsieh, Liang Huang, Seth Huggins, Min Liu, Michael A. Lovette, Kyle Quasdorf, William Powazinik, Jonathan Reifman, Jo Anna Robinson, Rahul P. Sangodkar, Sonika Sharma, Srividya Sharvan Kumar, Austin G. Smith, Gabrielle St-Pierre, Jason S. Tedrow, Oliver R. Thiel, Jonathan V. Truong, Shawn D. Walker, Carolyn S. Wei, Ashraf Wilsily, Yong Xie, Ning Yang, Andrew T. Parsons,
Org. Process Res. Dev. 2022.
https://doi.org/10.1021/acs.oprd.2c00249