Mannich-type reactions can provide straightforward access to nitrogen-containing products via the addition of a nucleophilic species to an imine. The decarboxylative Mannich reaction, where a carboxylic acid is used as a precursor of the nucleophile, is a useful variation of this type of reaction. This approach is attractive due to the only byproduct being CO2, but is usually restricted to imines bearing an electron-withdrawing group on the nitrogen atom.
Marc Presset and colleagues, Université Paris-Est Créteil, Thiais, France, have extended the use of decarboxylative Mannich reactions to N-alkyl aldimines, which react with substituted malonic acids half oxyesters (SMAHOs, general reaction pictured below). This reaction provides direct access to secondary β2,3-aminoesters. The team used DABCO (1,4-diazabicyclo[2.2. 2]octane) as a catalyst, TMSCl (trimethylsilyl chloride) and AcOH as additives, and bulk toluene as the solvent. The reactions were performed at 75 °C.
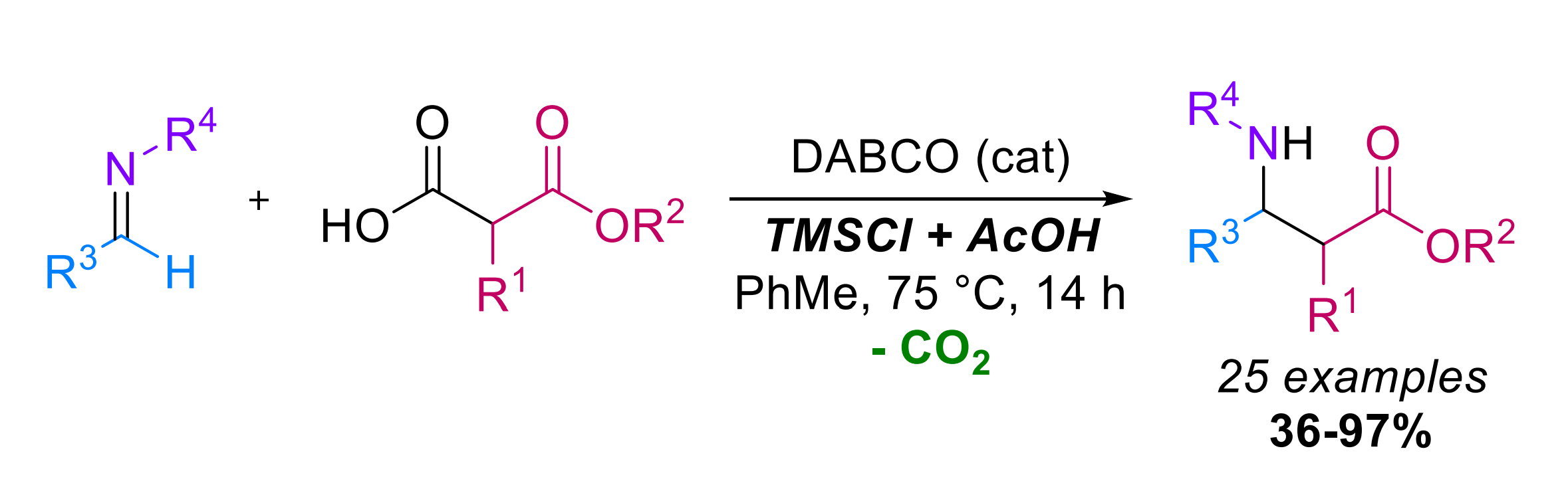
Using this approach, the researchers converted a broad range of imines and SMAHOs to the desired products with yields of 36–97 %. The acidic additives suppress olefination side reactions. The team also showed that a multicomponent approach can be used, in which the imine is generated in situ from aldehydes and amines. For this type of reaction, they changed the solvent to tetrahydrofuran (THF) and the reaction temperature to 65 °C. Overall, the transformation can be an attractive route for the preparation of secondary β-aminoesters.
- Decarboxylative Mannich Reactions with N‐Alkyl Imines,
Marine Pinaud, Leïla Vaïtilingom, Gayathiri Gnanalingam, Tania Xavier, Erwan Le Gall, Marc Presset,
Eur. J. Org. Chem. 2023.
https://doi.org/10.1002/ejoc.202300198
![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)


