Shunichi Fukuzumi and co-workers, Osaka University, Japan, have reported an efficient photocatalytic hydrogen-evolution system without the electron mediator methyl viologen, which is known to inhibit H2-evolution catalysts. They use the 9-mesityl-10-methylacridinium ion as the photocatalyst, dihydronicotinamide adenine dinucleotide as the electron donor, and water-soluble Pt nanoparticles (PtNPs) as the H2-evolution catalyst.
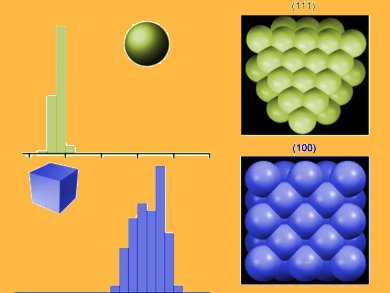
Cubic PtNPs (6.3 nm) exhibited the maximum rate of H2 generation. A substantial inverse kinetic isotope effect was seen in the rate of electron transfer, suggesting formation of the Pt–H bond is the rate determining step.
Hydrogen production from photocatalytic systems represents a clean source of energy and these mechanistic insights could lead to the development of high efficiency H2-gerenating systems based on Ni or Fe instead of precious metals.
- Size- and Shape-Dependent Activity of Metal Nanoparticles as Hydrogen-Evolution Catalysts: Mechanistic Insights into Photocatalytic Hydrogen Evolution
H. Kotani, R. Hanazaki, K. Ohkubo, Y. Yamada, S. Fukuzumi,
Chem. Eur. J. 2011.
DOI: 10.1002/chem.201002399