The stabilization of cationic reactive intermediates such as carbocations on π-basic aromatic surfaces is quite common. However, the complementary stabilization of anionic reactive intermediates and transition states on π-acidic aromatic surfaces does not occur in nature and, quite remarkably, has also been ignored in chemistry.
The first explicit example for such anion–π catalysis was reported four years ago by Stefan Matile and colleagues, University of Geneva, Switzerland [1]. The general principle has since been applied to several reactions, including enolate, enamine, and iminium chemistry, cascade processes, as well as the first reported anion–π enzyme.
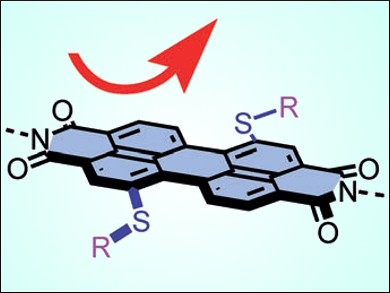
The team performed further studies and explored anion–π catalysis on the large and twisted π–acidic aromatic surfaces of perylenediimides (pictured), and observed giant quadrupole moments up to +71 Buckinghams (B), among the largest ever reported for organic molecules. Quadrupole moments perpendicular to the aromatic plane are a measure of a molecule’s π acidity; in comparison, TNT has around +20 B, benzene –9 B, and hexafluorobenzene +9 B.
Moreover, these highly π-acidic surfaces have sufficient space to comfortably accommodate larger transformations and thus afford the highest enantioselectivities reported to date for asymmetric anion–π catalysis.
- Asymmetric Anion-π Catalysis on Perylenediimides,
Chao Wang, François N. Miros, Jiri Mareda, Naomi Sakai, Stefan Matile,
Angew. Chem. Int. Ed 2016.
DOI: 10.1002/anie.201608842
Reference
- [1] Catalysis with Anion–π Interactions,
Yingjie Zhao, Yuya Domoto, Edvinas Orentas, César Beuchat, Daniel Emery, Jiri Mareda, Naomi Sakai, Stefan Matile,
Angew. Chem. Int. Ed. 2013, 52, 9940–9943.
DOI: 10.1002/anie.201305356
Correction (November 22, 2016)
Reference 1 originally misidentified the cited article.