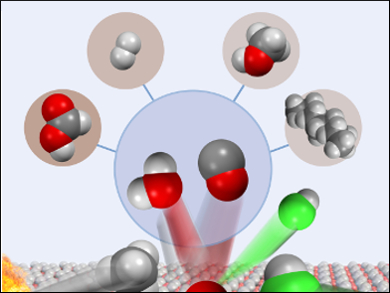
The transformation of methane into industrially valuable chemicals, such as carbon monoxide, is challenging because of the inert nature of C–H bonds [1]. C–H activation by oxychlorination uses hydrogen chloride and oxygen to form chloromethanes [2]. It can be coupled to a second oxidative dehydrohalogenation step to produce carbon monoxide and regenerate the acid. The industrial viability of this process hinges on these two steps linking up within a similar temperature and pressure regime to produce carbon monoxide continuously.
Javier Pérez-Ramírez, Swiss Federal Institute of Technology (ETH) Zurich, Switzerland, and colleagues have developed a robust gas-phase process to convert methane into carbon monoxide over a single vanadyl pyrophosphate (VPO) catalyst. A continuous flow fixed-bed reactor containing VPO and quartz particles operates for more than 100 hours under ambient pressure at 803 K. More than 90 % selectivity is retained for carbon monoxide without degradation of the catalyst.
Experimental observations suggest VPO is a discerning tandem oxidant, unsuited to direct methane activation but able to generate an auxiliary oxidant, chlorine, from hydrogen chloride. VPO catalyzes carbon monoxide formation from chloromethanes within the same temperature window. Carbon monoxide may, in turn, be incorporated into value-added chemicals such as formic acid and methanol, or serve as a feedstock for industrial processes such as the water–gas shift reaction or Fischer–Tropsch synthesis.
- Selective Production of Carbon Monoxide by Methane Oxychlorination over Vanadyl Pyrophosphate,
Vladimir Paunović, Guido Zichittella, Réne Verel, Amol P. Amrute, Javier Pérez-Ramírez,
Angew. Chem. Int. Ed. 2016.
DOI: 10.1002/anie.201608165
References
- [1] Understanding and exploiting C–H bond activation,
Jay A. Labinger, John E. Bercaw,
Nature 2002, 417, 507–514.
DOI: 10.1038/417507a - [2] Chlorinated Hydrocarbons,
M. Rossberg et al.,
in Ullmann’s Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2006.
DOI: 10.1002/14356007.a06_233.pub2
For more information, see this Research Highlight:
- A Flair for Methane Valorization,
Kim Meyer,
ChemViews Mag. 2016.
DOI: 10.1002/chemv.201600100