Photoelectrochemical water splitting under sunlight using a photocathode and a photoanode is a promising method for the production of hydrogen without emitting carbon dioxide. Chalcogenide-based photocathodes have previously shown remarkably large photocurrents arising from the hydrogen evolution reaction under simulated sunlight. In addition, collection of photoexcited carriers can be directly visualized by electron-beam-induced current (EBIC) analysis.
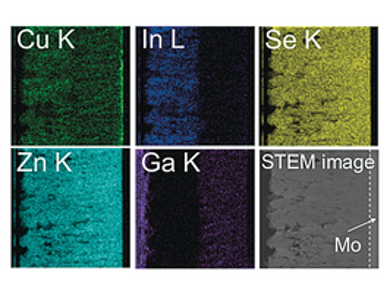
Kazunari Domen and colleagues at the University of Tokyo and the Japan Science and Technology Agency (JST) PRESTO, Tokyo, Japan, have developed a (ZnSe)0.85(CuIn0.7Ga0.3Se2)0.15 thin film photocathode that has a bilayer structure composed of Ga-rich and In-rich phases. This new photocathode produces a photocurrent that is almost twice that of a photocathode without the bilayer structure.
The researchers have also conducted cross-sectional EBIC mapping to obtain the distribution of charge separation, and found that a relatively uniform solid–liquid junction is formed in the case of the bilayer structure. This work demonstrates that efficient carrier collection from the light absorber is key for the creation of efficient photocathodes.
- Enhanced Hydrogen Evolution under Simulated Sunlight from Neutral Electrolytes on (ZnSe)0.85(CuIn0.7Ga0.3Se2)0.15 Photocathodes Prepared by a Bilayer Method,
Hiroyuki Kaneko, Tsutomu Minegishi, Mamiko Nakabayashi, Naoya Shibata, Kazunari Domen,
Angew. Chem. Int. Ed. 2016.
DOI: 10.1002/anie.201609202