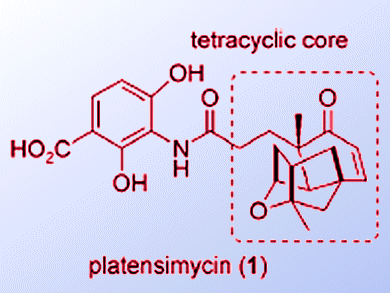
Platensimycin and platencin are FabF/H inhibitors; they inhibit bacteria’s ability to make the lipids used in cell membranes. They represent a new class of antibiotics, the development of which is becoming ever more important as bacterial resistance to antibiotics increases.
Reported syntheses of platensimycin are typically long (around 20 steps) and result in milligram yields. Jingxin Wang and Herman Sintim, University of Maryland, USA, have developed an approach to analogues of platensimycin and platencin that employs just two-flask reactions from commercially available aldehydes.
The 2,4-dihydroxybenzoic acid ester with a terminal amino group (1) can be synthesized on the gram-scale. A simple reductive amination with commercially available aldehydes or reactions with acid chlorides or sulfonyl chlorides gave platensimycin/platencin analogues in up to 69 % yield. The analogues were shown to be as effective as platensimycin in killing several bacterial strains.
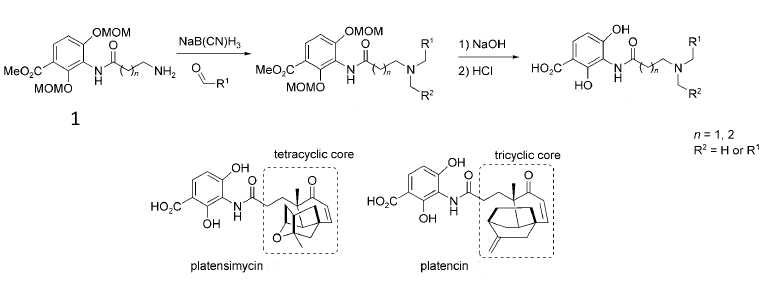
Image: (c) Wiley-VCH
- Dialkylamino-2,4-dihydroxybenzoic acids as Easily Synthesized Analogues of Platensimycin and Platencin with Comparable Antibacterial Properties
J. Wang, H. O. Sintim,
Chem. Eur. J. 2011.
DOI: 10.1002/chem.201002410