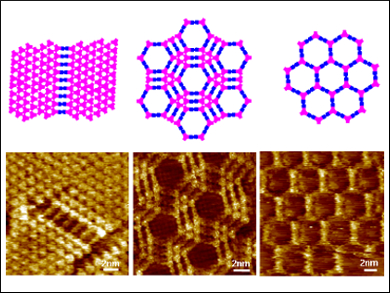
In covalent organic frameworks (COFs), molecular building blocks are arranged into 2D or 3D covalently linked porous macrostructures. They are robust materials with intrinsic porosity, crystallinity, and functionality. The synthesis of COF materials is mainly performed in solution, but a current focus lies on methods to synthesize COFs at interfaces. This could, for example, lead to single-layer 2D polymers.
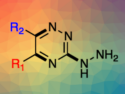
Shengbin Lei, Harbin Institute of Technology, China, and Tianjin University, China, and colleagues have studied the room temperature, surface-confined synthesis of boronic ester COF networks at the octanoic acid/ highly oriented pyrolytic graphite (HOPG) interface. The monomers used to form the framework were 4,4′-phenylazobenzoyl diboronic acid (ABBA) and 2,3,6,7,10,11-hexahydroxytriphenylene (HHTP).
A particular advantage of the on-surface condensation of boronic acids with diols at room temperature is that there can be no homopolymerization of boronic acids because this process requires higher temperatures. The researchers were able to show that the monomer concentration was the most important factor affecting the structures of the resulting frameworks.
By varying the concentration of both monomers, the team synthesized four kinds of regular, multiwall hexagonal porous structures, which could be identified in high-resolution scanning tunneling microscopy (STM). The dynamic nature of the boronic ester linkage at the interface could be observed in situ by using a voltage pulse. In addition, the team used 1H NMR spectroscopy to clarify the role of octanoic acid. The results indicate that the acid plays a role in accelerating the boronic esterification.
- Room-Temperature Synthesis of Covalent Organic Frameworks with a Boronic Ester Linkage at the Liquid/Solid Interface,
Chunhua Liu, Yanxia Yu, Wei Zhang, Qingdao Zeng, Shengbin Lei,
Chem. Eur. J. 2016.
DOI: 10.1002/chem.201603547