One of the priorities of the sustainable chemistry movement is to reduce and recycle chemical byproducts. One such byproduct is nitrogen dioxide, which is often produced in the chemical industry within procedures involving nitric acid, such as the nitration of aromatics. Although nitrified gas streams are commonly treated to produce reduced products (such as ammonia or urea), an attractive alternative is the re-utilization of NO2 as a precursor within organic synthesis, following the “waste to value” philosophy.
Markus Heinrich and colleagues, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany, have developed a two-step procedure to produce azobenzene-based pharmaceutical products using a mixture of air and NO2 inside a wet scrubber. The device was used to remove NO2 gas from the waste stream of a traditional diazotization reaction, which uses sodium nitrate and nitric acid.
This gas waste stream was then used to perform the same diazotization reaction, alongside aqueous HCl. The NO2 procedure produces competitive and in some cases better yields than the traditional diazotization procedure (100 % compared to 86 %), and was also able to circumvent a number of undesired side-reactions.
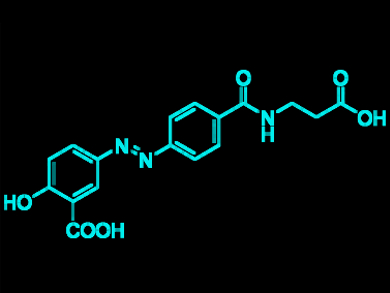
Following the formation of these diazonium salt precursors, azo coupling reactions were performed to produce known pharmaceutical products. In particular, balsalazide (an active compound against inflammatory bowel diseases, pictured) could be produced in 67 % yield using cheap organic bases in water at low temperature. This work showcases a method of waste and byproduct recycling which could be simply implemented within industrial chemistry. This approach could also be relevant in designing other ways of dealing with waste gas streams.
- Sustainable Synthesis of Balsalazide and Sulfasalazine Based on Diazotization with Low Concentrations of Nitrogen Dioxide in Air,
Dagmar Hofmann, Eva Gans, Jasmin Krüll, Markus R. Heinrich,
Chem. Eur. J. 2017.
DOI: 10.1002/chem.201605359