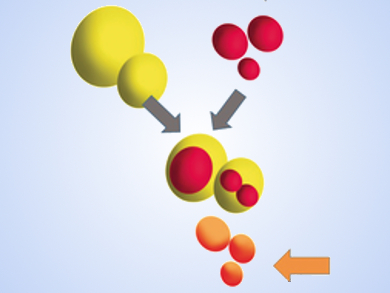
Biphasic reactions are commonplace. They are used everywhere from the pharmaceutical to the polymer industry. They have one major drawback that has to be overcome with each example – the need to separate and recycle the catalysts. They also suffer from thermal instability and high costs, but overcoming the former problem can make the latter two issues less relevant overall.
Xin Yan, Heyong Cheng, and Richard Zare, Stanford University, CA, USA, have found a way eliminate the phase-transfer catalysts from the equation. They have developed an efficient approach to carrying out two-phase reactions in colliding microdroplets propelled by a sheath gas – compressed air, nitrogen, or helium – which essentially does the job of the phase-transfer catalyst. This avoids the need for that substance to be separated out at the end of the reaction.
Oxidizing Proof
The team has demonstrated a proof-of-principle reaction using this system with various alcohols. These could be oxidized to the corresponding aldehydes or ketones within a few milliseconds in good yields (50 to 75 %). One particular example, the synthesis of 4-nitrobenzylaldehyde from 4-nitrobenzyl alcohol in the presence of sodium hypochlorite proved that scale-up is possible and reveals that the shearing sheath gas approach can greatly enhance synthetic effectiveness.
At the fundamental level, the immiscibility of two reagents might preclude their reaction as they would both remain in their respective solvent phases and so have little chance to react. A phase-transfer catalyst solves this problem by allowing one reagent to be carried into the phase of the second reagent and to facilitate a feasible reaction rate. The use of phase-transfer catalysts makes many otherwise untenable reactions possible but comes at a price, in terms of the drawbacks mentioned earlier.
Stirring Reactions
Commonly, the phase-transfer catalyst will allow the transport of a polar, or water-soluble, reactant into an organic solvent in which an apolar, hydrophobic reagent is dissolved. It might also work to simply bring the two disparate types of reagent together at the interface between the two phases. Often, vigorous magnetic or mechanical stirring is used to agitate the two phases and allow them to blend temporarily and so bring reactants into some sort of proximity. Similarly, ultrasonication or a rotor-stator homogenizer might be used to give a two-phase reaction a boost. However, all these approaches still rely on the presence of a phase-transfer catalyst.
Zare and his colleagues were aware of more conventional, single-phase reactions being carried out in microdroplets to improve their efficiency and yields. Microdroplets formed by spray-based ionization techniques, surface drop-casting, or microfluidics work well, the team reports. The details of how and why reactions occur more effectively in such microdroplets, rather than in the bulk phase, remains something of a mystery, however. Reactant confinement, evaporative processes, and other factors are all under consideration. It is likely that the truly important factor is the high surface-to-volume ratio of the system, the team suggests. The inner workings of such systems aside, the researchers turned to microdroplets to see whether or not the benefits might be extended to two-phase reactions.
Nebulizing Solutions
Essentially, the team atomized bulk reagent solutions using a turbulent blast of nebulizing nitrogen gas. Microscopic droplets of the two immiscible solvents are forced to collide, fuse, and mix, causing a reaction to take place. In their demonstration, it was possible to generate 1.2 milligrams of product per minute with an isolated product yield of 64 %. This “demonstrates the possible practical utility of the present method,” the team concludes. Future directions are to extend this process to other reactions and to demonstrate even larger scale-up.
“This approach is equivalent to a particle accelerator for organic synthesis because of the new opportunities that the technique creates,” Erick Carreira, Swiss Federal Institutes of Technology (ETH) Zurich, Switzerland, says. “Numerous advantages exist, which, as a synthetic chemist, I find quite exciting and compelling,” he adds. He points out that the technique avoids the use of phase-transfer catalysts and thus represents an advance in atom-economy. Secondly, the fact that the two reacting components are independently generated in separate streams is important because of the flexibility of the set-up. Thirdly, the fact that the reaction parameters, such as droplet size, method of preparation, speed, and details of collision can be fine-tuned provides for the versatility that is necessary for implementing this exciting new method in a host of other reactions. “For the modern researcher who is not afraid of thinking and working outside their comfort zone and is ready to adopt innovative, new approaches, the work of Zare lays out a new world of possibilities,” Carreira states.
- Two-Phase Reactions in Microdroplets without the Use of Phase-Transfer Catalysts,
Xin Yan, Heyong Cheng, Richard N. Zare,
Angew. Chem. Int. Ed. 2017.
DOI: 10.1002/anie.201612308