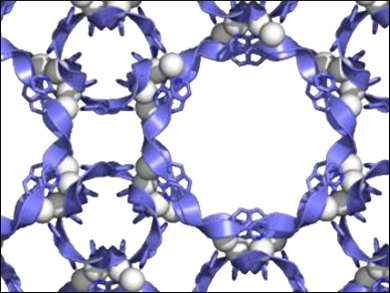
Concerted folding and assembly processes are essential for protein self-assembly but have rarely been used in synthetic chemistry. Researchers have been aiming to develop an artificial folding-and-assembly strategy easily construct well-defined peptide pores from a short peptide fragment and a metal ion.
Makoto Fujita, University of Tokyo, Japna, and colleagues have synthesized porous peptide complexes by complexation of AgNTf2 and tripeptide ligands with a Gly-Pro-Pro sequence. The network of the polyproline II helices, found in collagen, gave 1.5-nm-sized pores, which could be modified by introduction of a proline analogue or functional groups.
High-resolution crystal structures revealed that the polyproline II helix conformation has a fine balance of structural fidelity and flexibility upon pore modifications. The resulting peptide-based pores could be used for molecular recognition or reactions.
- Porous Peptide Complexes by a Folding-and-Assembly Strategy,
Tomohisa Sawada, Motoya Yamagami, Shuji Akinaga, Tatsuki Miyaji, Makoto Fujita,
Chem. Asian J. 2017.
DOI: 10.1002/asia.201700458