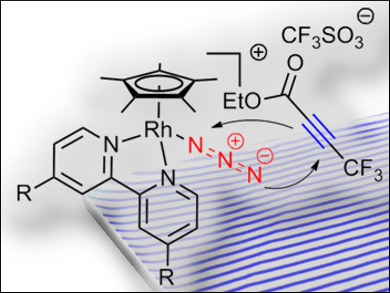
To specifically target individual residues in bio(macro)molecules, bioorthogonal coupling reactions that do not show any cross-reactivity with the regular constituents of biological systems are required. On the basis of the known reactivity of metal azide complexes with electron-poor alkynes, the cycloaddition of a series of rhodium azide complexes to a model alkyne was studied for this purpose.
Ulrich Schatzschneider, Julius-Maximilians-Universität Würzburg, Germany, and colleagues have prepared rhodium(III) azide half-sandwich complexes (pictured) that easily undergo cycloaddition reactions with ethyl 4,4,4-trifluoro-2-butynoate in acetonitrile at room temperature without a catalyst. The reactions afford triazolate products, which were simply obtained by precipitation in 65–75 % yield.
The trifluoromethyl group enabled monitoring of the reaction by 19F NMR spectroscopy. Kinetic studies were conducted with solution IR spectroscopy by following the decrease in the intensity of the signal for the azide stretching vibration. The researchers found that when electron-donating substituents were added at the 4,4′-positions of the 2,2′-bipyridine coligand, the pseudo-first-order rate constants became faster than those for the Staudinger ligation commonly used for bioconjugation reactions.
- Electronic Influences on the Stability and Kinetics of Cp* Rhodium(III) Azide Complexes in the iClick Reaction with Electron-Poor Alkynes,
Luisa Waag-Hiersch, Jan Mößeler, Ulrich Schatzschneider,
Eur. J. Inorg. Chem. 2017.
DOI: 10.1002/ejic.201700199