Transition-metal-catalyzed cross coupling is commonly used in organic synthesis for the production of fine chemicals. In particular, sp2–sp3 cross coupling has been a vibrant area of research, typically used for complex fragment coupling.
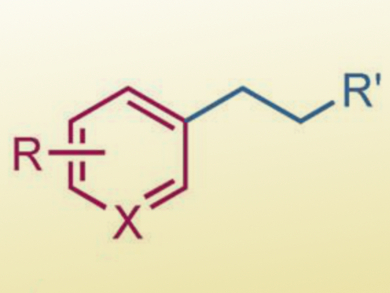
Stephen L. Buchwald, Massachusetts Institute of Technology (MIT), Cambridge, USA, and colleagues have developed a complementary method for sp2–sp3 cross coupling by using a combination of copper(I) hydride (CuH) and palladium catalysis. This method allows terminal olefins, a common and widely available class of chemicals, to couple with aryl halides by an anti-Markovnikov hydroarylation with high efficiency and regioselectivity (example product pictured).
The protocol has been shown to work for a broad range of substrates containing a variety of functional groups. Screening of cyclic alkenes also demonstrates that cyclopentene is an effective coupling partner under similar reaction conditions. Importantly, this method removes the need for stoichiometric metal reagents, which can often be air- and water-sensitive and require multistep synthetic routes.
- A Dual Palladium and Copper Hydride Catalyzed Approach for Alkyl-Aryl Cross-Coupling of Aryl Halides and Olefins,
Stig D. Friis, Michael T. Pirnot, Lauren N. Dupuis, Stephen L. Buchwald,
Angew. Chem. Int. Ed. 2017.
DOI: 10.1002/anie.201703400