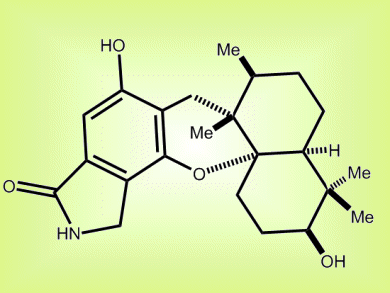
The worldwide spread of the H5N1 avian influenza and the recent outbreak of the new type of H1N1 human influenza have raised public concerns about global influenza pandemics. The adverse side effects and the virus’ resistance to current drugs continually drive the development of new anti-influenza agents. (+)-Stachyflin (pictured) has shown promising anti-influenza activity both in vitro and in vivo.
Tadashi Katoh and co-workers, Tohoku Pharmaceutical University, Japan, have reported the enantioselective total synthesis of (+)-stachyflin. The method used an innovative acid-induced cascade epoxide-opening/rearrangement/cyclization reaction to stereoselectively construct the requisite pentacyclic ring system in one step. The flexible cascade reaction could allow analogues stachyflin to be synthesized and their structure–activity relationships elucidated.
- Enantioselective Total Synthesis of (+)-Stachyflin: A Potential Anti-Influenza A Virus Agent Isolated from a Microorganism
J. Sakurai, T. Kikuchi, O. Takahashi, K. Watanabe, T. Katoh,
Eur. J. Org. Chem. 2011.
DOI: 10.1002/ejoc.201100173